Abstract
Asthma is a well-known inflammatory lung disease; however, the specific underlying mechanism is largely unknown. We previously demonstrated that alloferon effectively downregulates pulmonary inflammation. In this study, we examined whether alloferon has a therapeutic effect on asthma. Alloferon remarkably decreased the number of eosinophils, macrophages, and neutrophils in the bronchoalveolar lavage fluid (BALF) from ovalbumin (OVA)-induced asthma mice. It was synergistically decreased with 2.5 mg/kg prednisolone (PDA). Inflammatory cell infiltration around the bronchioles and in the alveolus of OVA-induced asthma mice was effectively prevented by alloferon alone and combined treatment with alloferon and PDS. The production of IL-5 and IL-17 was decreased by alloferon alone and combined treatment with alloferon and PDS. There was no change the level of total immunoglobulin (Ig) following alloferon administration; however, total Ig was decreased by PDS. IgG2a levels were not changed by either alloferon alone or alloferon in combination with PDS. However, the levels of OVA-specific IgG1 and IgE were decreased by alloferon and PDS. In conclusion, our results suggest that a combination of alloferon and prednisolone is effective for the treatment of asthma, as it prevents inflammatory cell infiltration via the downregulation of IL-5 and IL-17 production and decreases IgG1 and IgE production via the suppression of T helper type 2 immune response.
Although asthma is a well-known inflammatory lung disease, the specific underlying mechanism is largely unknown. Airway obstruction and epithelial fibrosis caused by airway remodeling are hallmarks of asthma, and asthma treatment is frequently dependent on the use of corticosteroids (12). However, long-term corticosteroid use is not recommended due to its adverse effects, such as suppression of the hypothalamic-pituitary axis, reduced bone growth in the young, and increased risk of opportunistic infections (3). In terms of the immune responses induced during the pathogenesis of asthma, it is known that T helper type 2 (Th2)-derived cytokines are closely related to the development and pathogenesis of asthma (45). Therefore, Th2 cytokines, such as IL-4, IL-5, and IL-13, are useful targets for asthma therapy (6). In fact, a beneficial therapeutic effect has been demonstrated with an IL-4 antagonist (7). In addition, neutralization of IL-5 by specific antibodies effectively reduced eosinophilic inflammation and airway hyper-responsiveness (89). IL-13 regulates IgE production and functions similar to IL-4 (10). These results suggest that suppression of Th2 cells and stimulation of Th1 via regulation of Th1-Th2 balance is a potential therapeutic pathway for asthma. Nakajima et al. recently reported the role of IL-17 and IL-23 in airway inflammation in asthma (11). Among the six IL-17 forms (IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F), mainly IL-17A and IL-17F are produced by Th17 cells and are involved in the neutrophil infiltration observed in the murine asthma model (1213). In addition, IL-23 is an essential factor for the maintenance of Th17 cells and their function (1415).
Alloferon is a 13-amino acid peptide that was first isolated from an insect immune system (16). It was reported to show anti-tumor effects via upregulation of NK cell activity, and anti-viral effects, especially against herpes virus, through regulation of the viral life cycle (1718). It was also recently reported that alloferon effectively downregulates the production of proinflammatory cytokines, such as IL-6, IL-8, and TNF-α, in UVB-induced skin inflammation (19). We also showed that alloferon alleviates dextran sulfate sodium-induced colitis via downregulation of IL-6 and TNF-α (20). Based on its immune-modulating activity, it seems that alloferon shows anti-tumor, anti-viral, and anti-inflammatory effects.
Since asthma can be effectively controlled by regulating the Th1-Th2 balance and alloferon has immune-modulating activity, we hypothesized that alloferon might be an effective therapeutic agent for asthma. Therefore, in the present study, we investigated the anti-asthmatic effect of alloferon in an ovalbumin (OVA)-induced murine asthma model.
Eight-week-old female BALB/c mice were purchased from Orient Bio (Seoul, Korea). Animals were housed in a temperature-controlled room (24±3℃) under a 12-hr light/dark cycle in the animal facility of Seoul National University College of Medicine. Food and water were provided ad libitum. Animals were cared for and handled in accordance with the guidelines of the SOP of our institute, and the study protocol was approved by the Institute of Laboratory Animal Resources of Seoul National University.
OVA (Grade V) was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was detoxified using a DetoxiGel column (Pierce, New York, USA) and quantified using the BCA method. One hundred microliters of phosphate buffered saline (PBS) or an emulsion containing 100 µg of OVA and 2 mg of alum was injected intraperitoneally for three consecutive days. Two weeks later, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and rompun (10 mg/kg), and then they received intranasal instillations of 30 µL of PBS containing 25 µg of OVA for two consecutive days. Three days later, the intranasal instillation was administered again for two consecutive days. Alloferon (2 mg/kg) and/or prednisolone (5 mg/kg) were intraperitoneally injected for six consecutive days. Alloferon is solid-phase synthesis technique by Any-Gen Co., Ltd. (Gwangju, Korea) and supplied by Allotech Co., Ltd.. The purity of the synthetic peptide, as measured by HPLC is 98% or more.
Airway responsiveness was measured using a whole body plethysmograph (OCP 3000; Allmedicus, Kyunggi-do, Korea) on day 25. Mice were exposed to 0, 12.5, 25, and 50 mg/mL methacholine (Sigma-Aldrich) using a Ultrasonic nebulizer (PARI, Starnberg, Germany) for 150 seconds at each concentration. After placing the mouse in the chamber, airway responsiveness was measured by monitoring enhanced pause (Penh) for 150 seconds. Penh was calculated by the following equation: Penh=(Te/RT-1)×PEF/PIF, where Te is expiration time (sec), RT is relaxation time (sec), REF is the peak expiratory flow rate (mL/s), and IF is the peak inspiratory flow rate (mL/s).
Mice were sacrificed with a lethal dose of ketamine and rompun. BALF was obtained by administering 1 mL of sterile PBS. The BALF was centrifuged at 4,000 rpm for 10 min. Supernatants were stored at 4℃, and then Th1 and Th2 cytokine levels were assessed with a cytokine bead array kit (BD, San Jose, CA, USA) according to the manufacturer's instructions. To count the cells in BALF, diluted BALF was spun down onto a glass slide. Each slide was dried and stained with Wright's Giemsa and examined under a light microscope.
Serum levels of OVA-specific total immunoglobulin (Ig) as well as IgE, IgG1, and IgG2a were measured by ELISA. Briefly, 96-well plates (Nunc, Rochester, NY, USA) were coated with 50 µg/mL detoxified OVA dissolved in blocking solution (1% skim milk and 0.05% Tween 20 in PBS) at RT for 3 hr, and then at 4℃ overnight. After the plates were washed, blocking solution was added, and the plates were incubated at RT for 1 hr. After washing, 100 µL of serially diluted serum was added to each well, and the plates were incubated at RT for 2 hr. The serum added to the first well was diluted 1:50 with blocking solution. To obtain calculation curves, OVA-specific reference serum, which was acquired from another experiment, was serially diluted and added to each plate. After washing, alkaline phosphatase-conjugated anti-mouse Ig was added, and the plates were incubated at RT for 1 hr. After incubation, p-NPP (Amresco, Solon, OH, USA) was added. The optical density (OD) at 405 nm was measured using a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The secondary antibodies used were goat antimouse polyvalent Ig (1:1,000), goat anti-mouse IgG1 antibody (1:1,000), goat anti-mouse IgG2a antibody (1:1,000), and rat anti-mouse IgE antibody (1:1,000).
The lungs and trachea were resected and fixed overnight in 10 mL of Bouin's solution. Specimens were dehydrated, fixed, embedded in paraffin wax, and then cut into 5-µm thick sections. The sections were placed on glass, stained with hematoxylin and eosin, and examined under a light microscope (OLYMPUS, Shinjuku-ku, Japan).
Data are presented as the mean±SD. An unpaired two-tailed t-test was used to compare groups [WT vs. alloferon (Allo) or prednisolone (PDS) vs. Allo+PDS]. P values less than 0.05 were considered statistically significant. All statistical tests were carried out using GraphPad InStat version 5.01 (GraphPad Software, Le Jolla, CA, USA).
To examine the effect of alloferon on airway resistance, airway hyper-responsiveness was measured by whole body plethysmograph after administration of methacholine (6.25, 12.5, 25, and 50 mg/mL). We observed that airway resistance was increased by treatment with OVA (12.59±0.65). We used PDS as a positive control to compare to alloferon. Airway resistance was decreased by treatment with PDS (2.5 mg/kg, 9.62±0.65; 5 mg/kg, 8.22±0.59), but was not decreased by treatment with alloferon (11.04±1.34). In addition, there was no synergistic or additive effect of treatment with alloferon and PDS (Fig. 1A). There were no changes in body weight following treatment with alloferon and/or PDS (Fig. 1B).
We obtained BALF and counted the number of cells in BALF to determine the effect of alloferon on inflammatory cell infiltration into the lungs. As shown in Fig. 2A, the number of cells in BALF from alloferon-treated mice was remarkably lower than that from untreated OVA-induced asthma mice. The number of cells in alloferon-treated mice was nearly identical to the number in mice treated with 2.5 mg/mL PDS. To investigate cell composition, the immune cells were stained with Wright's Giemsa and counted. The results showed that eosinophil and macrophage infiltration was effectively inhibited by treatment with alloferon, and infiltration was synergistically inhibited by 2.5 mg/mL PDS (Fig. 2B). Neutrophil infiltration was also effectively suppressed, although to a lesser degree. Then, we conducted a histological examination of the lungs from each experimental mouse. In mice with OVA-induced asthma, inflammatory cell infiltration around the bronchioles and into the alveolus, airway remodeling with peribronchiolar smooth muscle hypertrophy, and epithelium hyperplasia were observed (Fig. 3B). These were effectively prevented by treatment with alloferon and combined treatment with alloferon and PDS (Fig. 3C-G).
Next, we measured the levels of Th2 cytokines in BALF from mice with OVA-induced asthma with and without alloferon treatment. When mice were challenged OVA, we found that the production of IL-1α, IL-5, IL-6, and IL-17 was increased (Fig. 4). We observed that IL-1α production was not decreased by alloferon treatment or combined treatment with alloferon and PDS. Although IL-6 production was slightly decreased following the treatment with alloferon, it was not further decreased by combined treatment with alloferon and PDS. However, the production of IL-5 and IL-17 was not only decreased by alloferon treatment but also further decreased by combined treatment with alloferon and PDS. Unexpectedly, there was no change in IL-4 and IFN-α production.
It is generally known that serum IgE level is remarkably increased in asthma. In addition, IgG1 is a Th1-dependent isotypes and IgG2a is a Th2-dependent isotype. Therefore, we examined the OVA-specific antibody isotypes present in serum of OVA-induced asthma following treatment with alloferon. There was no change in total Ig levels following treatment with alloferon; however, total Ig levels were decreased following treatment with PDS (Fig. 5A). IgG1 levels were not changed by treatment with alloferon or by combined treatment with alloferon and PDS (Fig. 5B). IgG2a and IgE levels were remarkably decreased by treatment with PDS; however, we did not detect a synergistic effect between alloferon and PDS (Fig. 5C and D). However, we found that the levels of OVA-specific IgE in serum of OVA-induced asthma tends to decrease by treatment with alloferon or its combined treatment with PDS (Fig. 5D).
There have been several asthma treatment trials based on modulation of immune responses (78910). However, administration of corticosteroids is still regarded as the best way to treat asthma, even though there are reports of adverse effects, including suppression of the hypothalamic-pituitary axis, reduced bone growth in the young, osteoporosis, and opportunistic infections (3). Therefore, an agent that can replace corticosteroids, even partially, should provide new insights into asthma treatment. We previously showed that alloferon effectively downregulates pulmonary inflammation and pulmonary fibrosis (unpublished data). In the present study, we examined whether alloferon has a preventive or therapeutic effect on asthma.
As IL-4, IL-5, and IL-13 play an important role in the development and progression of asthma, the pathogenesis of allergic asthma is mediated by the Th2 immune response (456). Thus, we expected that IL-4 and IL-5 production would be increased in OVA-induced asthma mice and that production would be decreased by treatment with alloferon, PDS, or combined treatment with alloferon and PDS. Although we did not detect any change in IL-4 production, we confirmed increased IL-5 production in OVA-induced asthma mice and decreased production following treatment with alloferon, PDS, and combined treatment with alloferon and PDS (Fig. 4). It is known that the migration, activation, growth, differentiation, and survival of eosinophils are regulated by IL-5 (21). We also observed a decreased number of eosinophils in BALF (Fig. 2) and lung tissues (Fig. 3). Taken together, these results show that alloferon exerts its anti-asthmatic effect via downregulation of IL-5 production and eosinophil infiltration.
The most important finding in our study is the downregulation of IL-17 production. IL-17 is a pro-inflammatory cytokine that is produced by Th17 cells and is involved in the infiltration of inflammatory cells, such as neutrophils, macrophages, and eosinophils (22). It is thought that IL-17A and IL-17F are important for the neutrophil infiltration observed in the murine asthma model (1213). It has also been reported that IL-17 deficiency is closely related to the impaired neutrophilic response and the reduction of airway remodeling (23). The number of neutrophils in BALF was decreased by alloferon treatment, and this decrease might be mediated by downregulation of IL-17 production. As we previously stated, the purpose of this study was to determine whether alloferon could be used for the treatment of asthma as a replacement for corticosteroids. It is known that corticosteroids decrease IL-17 production in severe asthma (24). Therefore, it seems that alloferon could, at least partially, replace corticosteroids. Thus, the adverse effects of corticosteroid treatment might also be reduced by treatment with alloferon or combined treatment with alloferon and corticosteroids.
Since corticosteroids are potent anti-inflammatory agents, the levels of OVA-specific total Ig and IgE in serum were decreased by treatment PDS alone (Fig. 5D). OVA-specific IgG1 was also decreased, but there was no significant change in the levels of OVA-specific IgG2a. As shown in Fig. 5C and D, PDS decreased OVA-specific IgG2a and IgE production, but we could not observe the effect of alloferon on the decrease of IgG2a and IgE production with statistical significance. Combined treatment of alloferon with PDS also didn't decrease of IgG2a and IgE production, but we found that the levels of OVA-specific IgE in serum of OVA-induced asthma tends to decrease by the treatment of alloferon and its combined treatment with PDS (2.5 mg/kg) (Fig. 5D). Therefore, further extensive study regarding for the therapeutic efficacy of alloferon with PDS via down-regulation of IgE production should be needed. In conclusion, our results suggest that a combination of alloferon and prednisolone is effective for the treatment of asthma, in terms of the prevention of inflammatory cell infiltration through downregulation of IL-5 and IL-17 production.
Figures and Tables
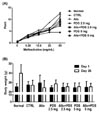 | Figure 1Alloferon does not affect airway resistance in OVA-induced asthma mice. (A) Plethysmography of experimental mice (n=6 per group) was measured after inhalation of an aerosol containing methacholine (6.25, 12.5, 25, and 25 mg/mL). Breathing was recorded for 150 sec as described in the Materials and Methods. Data are presented as the mean±SD. (B) Weight of the mice was measured on days 1 and 26 to monitor for possible adverse effects of alloferon and prednisolone administration. Data are presented as the mean±SD. |
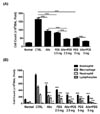 | Figure 2Alloferon reduced eosinophil infiltration into the lungs of mice with OVA-induced asthma. Bronchoalveolar lavage fluid (BALF) was obtained, and the number of cells in BALF was counted to determine the effect of alloferon on inflammatory cell infiltration as described in the Materials and Methods. (A) The total number of cells in BALF was measured using a Trypan blue dye exclusion assay. Data are presented as the mean±SD. ***p< 0.0001 (B) The cell composition in BALF with and without alloferon treatment was determined after Wright's Giemsa staining. Cells were collected from BALF by centrifugation and resuspended in 300 µL of PBS for cytospin preparation. Then, the composition of the cell fraction was determined after Wright's Giemsa staining. Data are presented as the mean±SD. *p<0.05, **p<0.001, ***p<0.0001. |
 | Figure 3Alloferon prevented airway remodeling in OVA-induced asthma mice. Lung and tracheal tissues were stained with hematoxylin and eosin as described in the Materials and Methods. The stained tissues were examined under a light microscope at 200×magnification. (A) Without OVA, (B) With OVA, (C) OVA+Alloferon (2.5 mg/kg), (D) OVA+Prednisolone (2.5 mg/kg), (E) OVA+Prednisolone (5 mg/kg), (F) OVA+Alloferon (2.5 mg/kg)+Prednisolone (2.5 mg/kg), (G) OVA+Alloferon (2.5 mg/kg)+Prednisolone (5 mg/kg). |
 | Figure 4Alloferon suppressed IL-5 and IL-13 production OVA-induced asthma mice. BALF was obtained, centrifuged, incubated with fluorescence-tagged antibody-conjugated beads using the CBA assay kit, and analyzed by flow cytometry. Data are presented as the mean±SD. The concentration of Th1/Th2 cytokines in BALF was determined according to the manufacturer's protocol. *p<0.05, **p<0.001, ***p<0.0001. |
 | Figure 5Alloferon did not alter OVA-specific antibody production. Blood was collected from the intra-orbital plexus with a heparinized capillary. After centrifugation at 1,500 rpm for 5 min at 4℃, the serum was collected. The concentration of OVA-specific antibodies was measured by ELISA as described in the Materials and Methods. Titers are shown relative to that in standard serum. (A) Total Igs, (B) OVA-specific IgG1, (C) OVA-specific IgG2a, (D) OVA-specific IgE. **p<0.001, ***p<0.0001. |
ACKNOWLEDGEMENTS
This work was supported by the grant from Seoul National University Hospital (Grant #: 0420120320).
References
1. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003; 111:215–225.

3. Belvisi MG, Brown TJ, Wicks S, Foster ML. New Glucocorticosteroids with an improved therapeutic ratio? Pulm Pharmacol Ther. 2001; 14:221–227.

4. Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998; 50:515–596.
6. Barnes PJ. Novel approaches and targets for treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 160:S72–S79.

7. Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, Agosti JM, Garrison L. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999; 160:1816–1823.
8. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophil, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.

9. Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. 2001; 2:71–79.
10. Wills-Karp M. The gene encoding interleukin-13: a susceptibility locus for asthma and related traits. Respir Res. 2000; 1:19–23.

11. Nakajima H, Hirose K. Role of IL-23 and Th17 Cells in Airway Inflammation in Asthma. Immune Netw. 2010; 10:1–4.

12. Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003; 28:42–50.

13. Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005; 171:12–18.

14. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201:233–240.

15. McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007; 8:1390–1397.

16. Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, Platonov VG, Bulet P. Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA. 2002; 99:12628–12632.

17. Lee N, Bae S, Kim H, Kong JM, Kim HR, Cho BJ, Kim SJ, Seok SH, Hwang YI, Kim S, Kang JS, Lee WJ. Inhibition of lytic reactivation of Kaposi's sarcoma-associated herpesvirus by alloferon. Antivir Ther. 2011; 16:17–26.

18. Bae S, Oh K, Kim H, Kim Y, Kim HR, Hwang YI, Lee DS, Kang JS, Lee WJ. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology. 2013; 218:1026–1033.

19. Kim Y, Lee SK, Bae S, Kim H, Park Y, Chu NK, Kim SG, Kim HR, Hwang YI, Kang JS, Lee WJ. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol Lett. 2013; 149:110–118.

20. Kim H, Im JP, Kim JS, Kang JS, Lee WJ. Alloferon Alleviates Dextran Sulfate Sodium-induced Colitis. Immune Netw. 2015; 15:135–141.

21. Sitkauskiene B, Johansson AK, Sergejeva S, Lundin S, Sjostrand M, Lotvall J. Regulation of bone marrow and airway CD34+ eosinophils by interleukin-5. Am J Respir Cell Mol Biol. 2004; 30:367–378.

22. Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, Gagliardo R, Profita M, Bousquet J, Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages,and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999; 103:563–573.

23. Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyper-responsiveness. Am J Respir Crit Care Med. 2009; 180:720–730.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download