Abstract
Hematopoietic stem and progenitor cells (HSPCs) can produce all kind of blood lineage cells, and gut microbiota that consists of various species of microbe affects development and maturation of the host immune system including gut lymphoid cells and tissues. However, the effect of altered gut microbiota composition on homeostasis of HSPCs remains unclear. Here we show that compositional change of gut microbiota affects homeostasis of HSPCs using Rag1-/- mice which represent lymphopenic condition. The number and proportions of HSPCs in Rag1-/- mice are lower compared to those of wild types. However, the number and proportions of HSPCs in Rag1-/- mice are restored as the level of wild types through alteration of gut microbiota diversity via transferring feces from wild types. Gut microbiota composition of Rag1-/- mice treated with feces from wild types shows larger proportions of family Prevotellaceae and Helicobacterceae whereas lower proportions of family Lachnospiraceae compared to unmanipulated Rag1-/- mice. In conclusion, gut microbiota composition of lymphopenic Rag1-/- mice is different to that of wild type, which may lead to altered homeostasis of HSPCs.
Hematopoietic stem and progenitor cells (HSPCs) are multipotent cells that capable of producing all adult blood cells throughout life (12). Among HSPCs, HSCs (hematopoietic stem cells) have long-term reconstruction capacity and can reconstruct whole circulation system over the lifespan (34). Most of HSCs in the bone marrow require a dormant state to preserve their self-renewal capacity and to prevent stem cell exhaustion (567). Dormant HSCs stay on G0 state in cell cycle and have very low cell metabolism (4), thus these cells do not contribute to maintenance of the homeostasis of the circulation and the immune system in steady states (8). Otherwise, homeostatic HSCs differentiate once every 28~36 days, which is comparatively shorter than dormant HSPCs (39). Only homeostatic HSCs are capable of keeping circulation and immune system at homeostasis during steady state via hematopoiesis that the generation of blood cell lineages. During heavy bleeding or in infectious or irradiated state, dormant HSCs are awakened and turn into an injury-activated state. Injury-activated HSCs have very high metabolism and have continuous cell cycle to compensate for massive blood loss (4).
Human intestine is colonized by 100 trillion microorganisms, thus interaction with microbiota shapes host immune system (10111213). Although there are lots of evidences suggesting that gut microbiota affects host immune system, whether altered composition of gut microbiota affects the homeostasis of HSPCs remained unknown. In this study, we show that compositional change of gut microbiota can alter the number of HSPCs. Our results suggest that gut commensal microorganism community is one of the important factors for regulating homeostasis of HSPCs in the bone marrow.
Female C57BL/6 Rag-1-deficient mice (6 to 8 weeks of age) were purchased from the Charles River Breeding Laboratories (Japan). Mice were kept under specific pathogen free (SPF) conditions in animal care facility in POSTECH (Pohang, Republic of Korea). The procedure of animal experiment was approved by the Institutional Animal Care and Use Committee (IACUC) at the POSTECH Biotech Center.
Feces from wild type mice or colonic feces from H. muridarum mono-associated mice were diluted to 12 g/ml of PBS. Suspensions were placed for 3 min at room temperature. 400 µl of supernatant was injected to each Rag-1-deficient mice 2 times per week for 4 weeks by oral gavage.
Bone marrow was collected by flushing femurs with RPIM containing 2% FBS. Cells were stained with a combination of anti-lineage (CD3e, B220, Gr-1, CD11b, NK1.1, and MHC II) APC, anti-c-Kit PE-cy7, anti-CD150 PerCP-eFluor710, anti-CD48 PE (eBioscience, USA), and anti-Sca-1 BV421 (Biolegend, USA). Flow cytometry was performed on a FACS Fortessa (BD Bioscience, USA) and Canto II and the data were analyzed using Flow Jo 3.0 (Tree Star, Ashland, OR).
Each 2.5 g fecal samples were placed in FastDNA SPIN Kit lysing matrix E tubes. 978 ml of sodium phosphate buffer and 122 ml MT buffer were added to each tube and mixed. Tubes were centrifuged at 14,000 g, 10 min. Supernatant was transferred to new tubes and added 250 µl Protein Precipitation Solution (PPS) and shaken 10 times by hand. Tubes were centrifuged at 14,000 g, 5 min. Supernatant was transferred to new tubes and added 1,000 µl Binding Matrix Suspension buffer. Tubes were placed on rotator for 2 min. Tubes were placed on a rack for 3 min. 500 µl of supernatant were removed Remaining amount of supernatant were suspended and transferred to a SPIN FILTER and centrifuged at 14,000 g, 5 min. The catch tubes were emptied. 500 µl of SEWS-M were added to each tube and resuspended gently. Tubes were centrifuged at 14,000 g, 2 min twice. Catch tubes were replaced with new tubes. 50 µl of DNase/Pyrogen-free water were added to each tube and resuspended gently. Tubes were centrifuged at 14,000 g, 1 min. DNA in catch tubes were stored at -20℃. PCR was performed with Taq DNA polymerase and primers to the V1 - V3 region of the 16S rRNA gene. Then, the amplified DNA was used as template for 454 GS Junior (Roche Diagnosis, Indianapolis, IN) pyrosequencing. Filter-passed 3,000 reads were subjected to operational taxonomic unit (OTU) analysis with the cutoff similarity of 97% identity by using CLcommunity™ softwere (14).
Gut microbiota direct innate immune cell development via promoting hematopoiesis (15). In addition, Rag1-/- mice that lack all mature lymphocytes have different compositions of gut bacterial community compared to wild type mice (1617). To check the number and proportion of the HSPCs in response to change of commensal microbial composition via a lymphopenia, we measured population of the bone marrow HSPCs of C57BL/6 female wild type and Rag1-/- mice. The absolute number of total bone marrow cells was decreased in Rag1-/- mice than wild type (Fig. 1A). As assessed by flow cytometry, the absolute number and proportions of LSK cells (Lineage- Sca-1+ c-Kit+), LT-HSCs (Long-term HSCs; Lineage- Sca-1+ c-Kit+ CD150+ CD48-), and ST-HSCs (Short-term HSCs; Lineage- Sca-1+ c-Kit+ CD150+ CD48+) were dramatically reduced (Fig. 1B), while MPs (myeloid progenitors; Lineage- Sca-1- c-Kit+) were increased in the bone marrow of Rag1-/- mice (Fig. 1C and D). Similarly, percentage of ST-HSCs among LSK cells was reduced in the bone marrow of Rag1-/- mice (Fig. 1E). On the contrary, percentage of MPPs (multipotent progenitors; Lineage- Sca-1+ c-Kit+ CD150-CD48+) was relatively up-regulated among LSK cells. Taken together, lymphopenic condition reduces the number of LT-HSCs and ST-HSCs and raises the number of MPs within the bone marrow.
Gut microbial diversity in co-housed animals is equilibrated by coprophagy (18). To examine homeostatic change of the bone marrow HSPCs according to gut microflora alteration, we used an additional bacterial administration to mimic recolonization (Fig. 2A). We extracted gut bacteria of feces from the wild type mice, and then orally injected the bacterial extraction of the feces to Rag1-/- mice 4 times for 4 weeks. Another mice group was injected with the same volume of PBS as the bacterial injected Rag1-/- mice. As expected, oral gavage treatment of feces increase the absolute number of total bone marrow cells of the Rag1-/- mice (Fig. 2B). It also showed that the absolute number of LSK cells, LT-HSCs, ST-HSCs and MPPs were elevated as the level of wild types (Fig. 2C). Moreover, percentage of LSK cells, MPs, and MPPs among total bone marrow cells in gavaged Rag1-/- mice were increased than the wild types (Fig. 2D). Especially, a proportion of ST-HSCs in feces-treated Rag1-/- mice were significantly increased (Fig. 2D). Taken together, compositional change of gut microbiota in Rag1-/- mice via feces transference affects homeostasis of HSPCs within the bone marrow.
To determine whether bacterial transfer induces compositional change of gut microbial community, we obtained bacterial DNA sample from feces of the wild type and Rag1-/- mice. Bacterial compositions of the feces were analyzed by metagenomics analysis (Fig. 3A). It was shown that members of phylum Firmicutes were relatively less abundant and members of phylum Bacteriodetes had larger proportion in the feces-treated Rag-1-deficient mice than the PBS-treated Rag1-/- mice (Fig. 3B). Family Lachnospiraceae in phylum Firmicutes were mostly reduced and family Helicobacteraceae in phylum Proteobacteria and family Prevotellaceae in phylum Bacteroidetes were relatively increased (Fig. 3B). Next, we tried to find a species that had the most significant changed of the three phyla. We focused on family Helicobacteraceae that had only identified bacterial species among the most abundant top 15 species in total microbiota of the feces-treated Rag-1-deficient mice (Fig. 3C). These data suggest that gut microbiota composition in Rag1-/- mice could be altered by the injection of wild type mice gut bacteria.
In our metagenomics results, we found two species of bacteria, such as Helicobacter muridarum (H. muridarum) and Helicobacter ganmani (H. ganmani), which were abundant and identified. Since other bacterial species were unidentified or reduced in the feces-treated Rag1-/- mice, we focused on two Helicobacter bacteria. To examine whether these species of bacteria affect homeostasis of the host HSPCs in the bone marrow, we treated a single-species bacterial injection experiment using male C57BL/6 Rag1-/- mice. One group (5 mice) had been treated with H. muridarum via oral gavage 3 times for 4 weeks, while the other group (3 mice) had been treated PBS. Both groups were sacrificed at day 28 after first administration (Fig. 4A). Although absolute number of total bone marrow cells were significantly increased in bacteria-treated mice (Fig. 4B), the absolute number and proportions of HSPCs in the H. muridarum-treated mice were not up-regulated (Fig. 4C and D). The proportion of LT-HSCs, ST-HSCs, and MPPs among LSK cells had not shown significant change (Fig. 4E). These suggest that a single-species bacteria transfer of H. muridarum cannot induce the change in HSPCs homeostasis in the bone marrow in Rag-1-deficient mice.
The number and a proportion of HSPCs in Rag1-/- mice were down-regulated compared to wild types; however, Rag1-/- mice treated with wildtype feces restored the absolute number and the proportion of HSPCs in Rag1-/- mice as those of wild types. Feces treatment did not construct same composition of gut microbiota of Rag1-/- mice as that of wildtype mice. It would be difficult to equilibrate gut microbial diversity between wild types and Rag1-/- mice, even if bacteria from wild type are transferred and colonized on gut mucosa of Rag1-/- mice. Nevertheless, our data show that composition of wild types and feces-treated Rag1-/- mice share specific bacteria species. Interestingly, the most altered bacteria species were unidentified species. Furthermore, H. muridarum single colonization has no effect on controlling homeostasis of HSPCs in Rag1-/- mice. Therefore, our data indicate that restoration of the number and proportions of HSPCs are dependent on complex gut microbiota composition, not restricted to some of specific bacteria proportion. Inoculating several species of bacteria in Prevotellaceae and Helicobacterceae families in combination into Rag1-/- mice might be required for a future study. HSPCs might be affected by unknown signals from microbial community. Although defined mechanisms of regulating HSPCs by commensal gut microbiota is unknown, this result indicates that composition of gut microbiota have an effect on homeostasis of HSPCs.
In summary, gut microbiota affects the number and proportion of HSPCs, and compositional change of gut microbiota induces change of the homeostasis of HSPCs. Mechanisms of regulating homeostasis of HSPCs via gut microbiota, or specific bacterial signals that key regulators of hematopoiesis would be clearly established after further study.
Figures and Tables
 | Figure 1Lymphopenic condition reduces the number of HSPCs. (A) Absolute number of total bone marrow (BM) cells from wild type and Rag1-/- mice. (B) Flow cytometry of LSK cells (Lin- Sca-1+ c-Kit+) and MPs (Lin- Sca-1- c-Kit+), and LT-HSCs (Lin- Sca-1+ c-Kit+ CD150+ CD48-), ST- HSCs (Lin- Sca-1+ c-Kit+ CD150+ CD48+), and MPPs (Lin- Sca-1+ c-Kit+ CD150- CD48+). Absolute number (C) and the frequency (D) of LSK cells, MPs, LT-HSCs, ST-HSCs, and MPPs among total BM cells. (E) The percentage of LT-HSCs, ST-HSCs and MPPs among LSK cells. Mean values±s.e.m. are shown. *p<0.05, **p<0.01, ***p<0.001, compared with drinking water controls. (Student's t-test). |
 | Figure 2Feces delivery restores the number and proportion of HSCs in Rag1-/- mice. (A) Experimental scheme. (B) Absolute number of total BM cells from the mice groups. Absolute number (C) and the percentage (D) of LSK cells, MPs, LT-HSCs, ST-HSCs, and MPPs among total BM cells. (E) The percentage of LT-HSCs, ST-HSCs and MPPs among LSK cells. Mean values±s.e.m. are shown. *p<0.05, **p<0.01, ***p<0.001, compared with drinking water controls. (Student's t-test). |
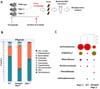 | Figure 3Feces delivery alters composition of colon microbial community. (A) Experimental scheme for metagenomics analysis. Gut microbiota composition based on phylum (A), family (B), and species (C) by bacterial DNA metagenomics analysis. Bacterial DNA in pooled feces (from 4 to 5 mice per each group) were identified by standard microbiological method. |
 | Figure 4Colonization of H. muridarum has no effect on HSCs of Rag1-/- mice. (A) Experimental scheme. (B) Absolute number of total BM cells from the mice groups. Absolute number (C) and the percentage (D) of LSK cells, MPs, LT-HSCs, ST-HSCs, and MPPs among total BM cells. (E) The percentage of LT-HSCs, ST-HSCs and MPPs among LSK cells. Mean values±s.e.m. are shown. *p<0.05, compared with drinking water controls. (Student's t-test). |
ACKNOWLEDGEMENTS
We thank Jaeu Yi and Charles D. Surh for providing materials and experimental techniques. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2044032).
References
1. Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003; 21:759–806.

2. Warr MR, Pietras EM, Passegue E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011; 3:681–701.

3. van der Wath RC, Wilson A, Laurenti E, Trumpp A, Lio P. Estimating dormant and active hematopoietic stem cell kinetics through extensive modeling of bromodeoxyuridine label-retaining cell dynamics. PLoS One. 2009; 4:e6972.

4. Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010; 10:201–209.

5. Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006; 6:93–106.

6. Arai F, Yoshihara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Suda T. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009; 1176:36–46.

7. Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008; 8:290–301.

8. Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015; 518:542–546.

9. Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008; 135:1118–1129.

10. Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013; 11:227–238.

11. Lederberg J, Mccray A. 'Ome Sweet 'Omics--A Genealogical Treasury of Words. The Scientist. 2001; 17(7):
12. NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. The NIH Human Microbiome Project. Genome Res. 2009; 19:2317–2323.

13. Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014; 14:827–835.

14. Park SJ, Namkoong H, Doh J, Choi JC, Yang BG, Park Y, Chul SY. Negative role of inducible PD-1 on survival of activated dendritic cells. J Leukoc Biol. 2014; 95:621–629.

15. Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014; 15:374–381.

16. Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. Host adaptive immunity alters gut microbiota. ISME J. 2015; 9:770–781.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download