Abstract
Bone marrow-derived mesenchymal stem cells (MSCs) have immunomodulatory properties and can suppress exaggerated pro-inflammatory immune responses. Although the exact mechanisms remain unclear, a variety of soluble factors are known to contribute to MSC-mediated immunosuppression. However, functional redundancy in the immunosuppressive properties of MSCs indicates that other uncharacterized factors could be involved. Galectin-9, a member of the β-galactoside binding galectin family, has emerged as an important regulator of innate and adaptive immunity. We examined whether galectin-9 contributes to MSC-mediated immunosuppression. Galectin-9 was strongly induced and secreted from human MSCs upon stimulation with pro-inflammatory cytokines. An in vitro immunosuppression assay using a knockdown approach revealed that galectin-9-deficient MSCs do not exert immunosuppressive activity. We also provided evidence that galectin-9 may contribute to MSC-mediated immunosuppression by binding to its receptor, TIM-3, expressed on activated lymphocytes, leading to apoptotic cell death of activated lymphocytes. Taken together, our findings demonstrate that galectin-9 is involved in MSC-mediated immunosuppression and represents a potential therapeutic factor for the treatment of inflammatory diseases.
Mesenchymal stem cells (MSCs) are non-hematopoietic adult stem cells typically isolated from adult tissues including bone marrow (BM), adipose tissue, and cord blood (123). Although MSCs are multipotent and can differentiate into various mesenchymal cell types, their immunomodulatory properties distinguish them from other types of stem cells such as hematopoietic stem cells (HSCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs). It has become more evident that MSCs possess immunomodulatory properties in vitro and in vivo, as demonstrated by suppression of a broad range of innate and adaptive immune cell types including lymphocytes, natural killer cells, neutrophils, macrophages, and dendritic cells (4). Furthermore, a number of studies have shown that MSCs improve immunological or inflammatory pathologies such as graft-versus-host disease, pancreatitis, and diabetes (567). The immunosuppressive properties of MSCs have already been applied in the development of stem cell therapies for treatment of inflammatory and autoimmune diseases (89). However, the precise molecular mechanisms involved in MSC-mediated immunomodulation remain incompletely understood. Although the cellular and molecular mechanisms of MSCs associated with inflammatory conditions are complex, a variety of soluble factors are critical for MSC-mediated immunomodulation (410). These secreted soluble factors that have been demonstrated to mediate MSC-associated immunosuppression include both protein factors such as transforming growth factor-β (TGF-β), indoleamine 2,3-dioxygenase (IDO), interleukin-10 (IL-10), and hepatocyte growth factor (HGF), and non-protein factors such as prostaglandin E2 (PGE2) and nitric oxide (NO) (11-13). However, blocking any of these soluble factors including TGF-β, IL-10, and HGF failed to achieve a complete ablation of MSC-mediated immunosuppression (14). This functional redundancy suggests that there could be unidentified factors regulating MSC-mediated immunosuppression under different pathological conditions.
Galectins belong to a phylogenetically conserved mammalian family of β-galactoside-binding lectins with a characteristic carbohydrate recognition domain (15). Galectins have pleiotropic functions in the immune system (1617). For example, galectin-9 was first identified as a chemoattractant and activator of eosinophils (1819). Evidence has accumulated to indicate that galectin-9 plays pivotal roles in the modulation of innate and adaptive immunity as well as in a broad spectrum of cellular responses, including cell adhesion, aggregation, apoptosis, and cytokine secretion (20212223). Galectin-9 dampens lymphocytic immune responses by preferentially inducing apoptosis of activated T helper 1 (Th1) and T helper 17 (Th17) cells (2324). In addition, it induces differentiation of naïve T cells into regulatory T cells (Tregs) and concomitantly it suppresses differentiation of T helper 17 cells (Th17) (2526). Furthermore, human galectin-9 has been shown to improve acute graft-versus-host disease in mice (27).
It has become increasingly apparent that the immunomodulatory effects of galectins resemble those of MSCs, suggesting that galectins could be a major MSC-derived factor involved in the suppression of inflammatory responses. This notion is supported by reports that galectin-1 and galectin-3 contribute to MSC-mediated T cell suppression (2829). However, little is known about the functional roles of galectin-9 in the immunosuppression elicited by MSCs. In this study, we examined whether galectin-9 is involved in human MSC-mediated immunosuppression. The functional roles of galectin-9 were evaluated by examining its ability to inhibit mitogen-stimulated lymphocyte proliferation through recombinant protein treatment and knockdown approaches. Our results showed that galectin-9 is induced in human MSCs by pro-inflammatory stimuli and contributes to the suppression of lymphocyte activation mediated by MSCs.
Bone marrow aspiration was approved by the Inha University Hospital Institutional Review Board (IRB) and written informed consent was obtained from a healthy donor (IRB number #10-51). Highly homogeneous clonal MSCs were isolated from BM as described previously (30). Markers of MSCs were determined by flow cytometry using a number of specific antibodies, including CD14 (BD Biosciences, San Diego, CA, USA), CD29 (Serotech, Kidlington, UK), CD31 (Serotec), CD34 (BD Biosciences), CD44 (Serotec), CD45 (BD Biosciences), CD49f (BD Biosciences), CD73 (BD Biosciences), CD90 (BD Biosciences), CD105 (Serotec), CD106 (BD Biosciences), CD133 (BD Biosciences), CD166(Serotec), HLA-DR (BD Biosciences), HLA-Class I (BD Biosciences), CXCR4 (BD Biosciences), and Oct-4 (Cell Signaling Technology, Danvers, MA, USA). MSCs were positive for CD29, CD44, CD49f, CD73, CD90, CD105, CD166, HLA-Class I, and Oct-4 but were negative for C14, CD31, CD34, CD45, CD106, CD133, CXCR4, and HLA-DR (data not shown). For the assessment of differentiation potential, adipogenic, osteogenic, and chondrogenic differentiations were induced as described (6). MSCs isolated were successfully differentiated into these 3 mesenchymal cell types, indicating the multilineage differentiation potential (data not shown).
In order to examine intracellular galectin-9 induction, MSCs were treated with 10 ng/ml TNF-α (BD Bioscience) and 20 ng/ml IFN-γ (BD Bioscience) for the indicated times. After treatment, containing MSCs were washed in phosphate-buffered saline (PBS) containing 0.5% fetal bovine serum (FBS), fixed/permeabilized for 30 min. Then the cells were incubated with fluorescein isothiocyanate (FITC) anti-human galectin-9 antibody (BioLegend, San Diego, CA, USA) for 30 minutes at 4℃ followed by analysis in a flow cytometer (FACSCalibur; BD Biosciences). For analyzing TIM-3-expressing CD3-positive lymphocytes, anti-TIM-3 conjugated with Alexa Fluor 488 (R&D Systems, Minneapolis, MN, USA) and anti-human CD3 conjugated PerCP-eFluor 710 (eBioscience, San Diego, CA, USA) were used.
To determine the quality of galectin-9, TNF-a, or IL-17A in the conditioned medium, the wells in a culture plate was coated with 1 µg/ml anti-human galectin-9 antibody (R&D Systems), anti-human TNF-α antibody (BD Biosciences), or anti-human IL-17A antibody (BD Biosciences), respectively, for 24 h at 4℃. After blocking with blocking buffer (1% BSA in PBS), cell culture supernatant was incubated for 24 h at 4℃. After washing, the plate was incubated with a biotinylated anti-galectin-9 (0.2 µg/ml) for 1 h and was incubated with streptavidin-conjugated horseradish peroxidase (HRP) diluted in blocking buffer for 1 h. Then the absorbance at 450 nm of wavelength was measured in a microplate reader (Bio RAD, Foster City, CA, USA).
To isolate total RNA from MSCs, EasyBlue isolation reagent (Intron Biotechnology, Sungnam, Korea) was used according to manufacturer instruction. cDNAs was synthesized from 1 µg of total RNA by the AccuPower cDNA synthesis kit (Bioneer, Daejeon, Korea). The sequences of the primers used in this study was as follows; galectin-1 (Forward 5'-GGTCTGGTCGCCAGCAACCTGAAT-3' and Reverse 5'-TGAGGCGGTTGGGGAACTTG-3'), gaelcin-9 (Forward 5'-CAGGGCGGTTGGGGAACTTG-3' and Reverse 5'-TGAGGCAGTGAGCTTCACAC-3'), IDO (Forward 5'-GGGCAAATGCTATCATTGGAAAA-3' and Reverse 5'-GGTGGCCGGAGAAGAACATT-3') and GAPDH (Forward 5'-CCACTGGCGTCTTCACCAC-3' and Reverse 5'-CCTGCTTCA CCACCTTCTTG-3'). The PCR products were analyzed by resolution on 1% agarose gel followed by fluorescence image analyzer (LAS4000; Fuji Photo-Film, Tokyo, Japan).
Thymidine incorporation method was conducted to evaluate lymphocyte proliferation. Peripheral blood mononuclear cells (PBMCs) were collected from healthy donors (IRB #11-104) and separated by ficoll-hypaque density gradient centrifugation. To examine the effects of galectin-9 on the lymphocyte proliferation, 2×105 PBMCs stimulated with 1 µg/ml of phytohemagglutinin (PHA, Sigma-Aldrich, St. Louis, MO, USA) were treated with 1.5 µg/ml of recombinant human galectin-9 (rhGal-9; R&D Systems) for 3 days. In order to determine the immunosuppressive effects of MSCs, 2×105 PBMCs stimulated with 1 µg/ml of PHA were co-cultured with 4×104 MSCs (MSC to PBMC= 1:5) for 3 days. [3H]-thymidine (1 µCi/reaction) was added for the last 12 h of the culture. Lymphocyte proliferation rate was assessed as incorporated radioactivity in counts per minute (cpm).
CFSE assay was conducted to determine whether rhGal-9 or MSCs affect cell division of proliferating lymphocytes. Isolated PBMCs were labelled with 10 µM of CFSE (Invitrogen, Carlsbad, CA, USA) for 10 mins at 37℃. The cell were washed 3 times and re-suspended in the culture medium. The CFSE-labeled cells stimulated with PHA. After 5 days, the cells were harvested and analyzed by flow cytometry.
Galectin-9-specific siRNA oligomers (siGal-9; catalog number E-011319-00-0005) and scrambled oligomers (siCon; catalog number D-001950-01-05) were purchased from Dharmacon (GE Healthcare, Lafayette, CO, USA). siRNA transfection was performed according to manufacturer's instruction. Briefly, 5×103 MSCs were seeded onto a well of a 96-well plate. The next day, adherent MSCs were transfected with siGal-9 or siCon. The cells were further stimulated with 10 ng/ml TNF-α and 20 ng/ml INF-γ for 48 h. After knockdown of galectin-9 was confirmed by western blotting, the cells were subjected to in vitro immunosuppression assay and ELISA. For lentiviral shorthairpin RNA (shRNA)-mediated gene knockdown, BLOCK-iT Lentiviral RNAi Expression System was used purchased from Invitrogen. All processes including lentiviral cloning, packaging, production, and infection were performed according to manufacturer's instruction. Target sequences in human galectin-9 (NCBI reference NM_009587.2) are as follows; 5'-GGACTTCAGATCACTGT-3' and 5'-GGAAG ACACACATGCCTTTCC-3'.
Apoptosis was measured by using an Annexin V/PI detection kit (BD Biosciences) according to manufacturer's instruction. After PBMCs stimulated with PHA were incubated with 1.5 µg/ml of rhGal-9 or were co-cultured with MSCs for 3 days, they were harvested and re-suspended in 500 µl of binding buffer consisting of 0.1 M HEPES/NaOH (pH 7.4), 1.4 M NaCl, and 25 mM CaCl2. Then, the cells were stained with Annexin-V-FITC and PI for 30 min at 4℃. Apoptotic cells were determined by flow cytometry.
Inflammatory environments or reciprocal interactions between MSCs and activated immune cells are essential for inducing the immunosuppressive effects of MSCs (31). To generate an in vitro condition reflecting the pro-inflammatory environment, we treated human MSCs with the pro-inflammatory cytokines TNF-α and IFN-γ for 24 h. Vehicle-treated MSCs served as a control. We first examined mRNA expression of galectin-9 using RT-PCR. Cytokine treatment strongly induced galectin-9 and IDO in MSCs, while galectin-1 was constitutively expressed regardless of cytokine stimulation (Fig. 1A). Since galectins are expressed in the cytoplasm, flow cytometry was performed after cell permeabilization. Flow cytometric analysis demonstrated significant intracellular expression of galectin-9 in stimulated MSCs within 48 h (Fig. 1B). Galectins can also be secreted in a non-classical pathway bypassing the endoplasmic reticulum and the Golgi apparatus (32). In order to determine whether induced galectin-9 was secreted from MSCs, the culture supernatant was analyzed using ELISA. There was a significant accumulation of galectin-9 secreted from MSCs within 72 h (Fig. 1C). These results demonstrate that in the presence of pro-inflammatory stimuli, galectin-9 expression is highly induced in MSCs and its secretion is stimulated.
To examine the effects of galectin-9 on activated lymphocyte proliferation, PHA-activated PBMCs were treated with recombinant human galectin-9 (rhGal-9). Treatment with rhGal-9 significantly suppressed lymphocyte proliferation, similar to the inhibition observed for lymphocytes co-cultured with MSCs (Fig. 2A). CFSE assay also revealed that rhGal-9 treatment arrested cell division of activated lymphocytes (Fig. 2B). Thus, the functional effects of galectin-9 and MSCs were consistent, with similar inhibition of lymphocyte proliferation, demonstrating that galectin-9 could be an MSC-derived factor that elicits immunosuppression. Indeed, galectin-9 was secreted in higher amounts in the co-culture of MSCs and PHA-activated PBMCs (Fig. 2C). Release of TNF-α and IL-17A from activated PBMCs was correspondingly decreased by coculture of MSCs (Fig. 2C), suggesting that galectin-9 secreted from MSCs inhibit the production of pro-inflammatory cytokines.
To obtain direct evidence of galectin-9 involvement in MSC-mediated immunosuppression, we examined the effects of galectin-9 knockdown in MSCs on PHA-stimulated lymphocyte proliferation. Galectin-9 was knocked down by siRNA or shRNA-expressing lentivirus. MSCs were transfected with siRNA oligomers. Cells were incubated for 48 h followed by western blot analysis. Significant downregulation of galectin-9 protein expression by galectin-9-specific siRNA oligomers (siGal-9) was observed, while scrambled oligomers (siCon) did not modify galectin-9 protein expression (Fig. 3A). Galectin-9 secretion in the culture supernatant was also decreased as revealed by ELISA (Fig. 3B). Next, we examined the effects of siGal-9-treated MSCs on proliferation of PHA-stimulated lymphocytes. Interestingly, galectin-9 knockdown in MSCs did not suppress lymphocyte proliferation (Fig. 3C). In another experiment, we knocked down galectin-9 using a lentivirus expressing shRNA targeting galectin-9 (shGal-9). After cloning of the lentiviral shGal-9 construct, viral particles were produced in 293FT cells. A lentivirus expressing shLacZ served as a control. MSCs were infected with shGal-9-expressing or shLacZ-expressing lentiviral particles. Dramatic decreases in galectin-9 protein expression and secretion were confirmed by western blot analysis and ELISA, respectively (Fig. 3D). Consistent with the siRNA results, shGal-9-infected MSCs did not suppress proliferation of activated lymphocytes (Fig. 3E). Taken together, these gene knockdown experiments demonstrated that galectin-9 is involved in MSC-mediated immunosuppression. To characterize the the mode of action of galectin-9 in MSC-mediated immunosuppression, we examined whether MSCs or rhGal-9 treatment affect lymphocytes expressing T cell immunoglobulin and mucin-containing domain-3 (TIM-3), a receptor for galectin-9. PHA stimulation induced TIM-3-positive lymphocytes. In the presence of rhGal-9, TIM-3-positive lymphocytes were decreased in a dose-dependent manner (Fig. 4A). Similarly, co-culture of MSCs also reduced TIM-3-expressing lymphocytesin a cell number-dependent manner (Fig. 4A), suggesting that galectin-9 secreted from MSCs binds to TIM-3 to promote galectin-9/TIM-3 internalization. We further examined the effects of galectin-9 or MSCs on apoptosis of activated lymphocytes. In the presence of rhGal-9 or MSCs, cell death of the activated lymphocytes was significantly increased (Fig. 4B). Apoptotic cell death induced by rhGal-9 was comparable to that induced by MSCs, suggesting that galectin-9 contributes to MSC-mediated immunosuppression possibly by inducing cell death of activated lymphocytes.
Over the past decade, MSCs have emerged as a promising cell source for the treatment of inflammatory diseases because of their inherent immunomodulatory properties. A prominent feature of MSCs is their capacity to potently suppress T cell activation via multiple mechanisms; they are also able to modulate additional immune cell types including B cells, natural killer cells, dendritic cells, and macrophages (4). A number of studies have demonstrated that MSCs effectively inhibit proliferation, activation, and inflammatory cytokine secretion of activated effector T cells (333435) while they promote the expansion of Tregs (11). Moreover, they have been shown to treat or attenuate several experimental or clinical pathologies such as graftversus-host disease, colitis, pancreatitis, experimental autoimmune encephalomyelitis, and diabetes (36). It is generally accepted that the paracrine mechanism plays a key role in the MSC-induced therapeutic effects in vitro and in vivo, indicating the functional significance of soluble factors released from MSCs (37). Although several soluble factors have been suggested, the redundancy of the immunosuppressive pathways associated with MSCs raises the possibility that uncharacterized soluble factors contribute to the immunomodulation elicited by MSCs.
Although galectin family members have been shown to modulate various lymphocyte functions, including T cell development and activation, apoptosis (17), and cytokine secretion, these proteins have only recently been implicated in MSC-mediated immunomodulation (38). Indeed, there are similarities in the immunomodulatory properties of galectins and MSCs. Galectin-1 is known to be involved in the initiation, amplification, and resolution of inflammation (39). Galectin-1 induces proliferation inhibition, growth arrest, and apoptosis of activated immune cells (4041). Furthermore, galectin-1 suppresses IL-2 production and elevates IL-10 secretion (4243). These functions exerted by galectin-1 have been demonstrated by exogenous addition of a micromolar concentrations of recombinant galectin-1 to T cells. Galectin-3 has pleiotropic functions and can promote apoptosis depending on intracellular or extracellular localization. Extracellular galectin-3 can accelerate activated T cell death whereas intracellular galectin-3 protects cells from apoptosis and promotes T cell proliferation (4445). Exogenous addition of galectin-3 was found to directly initiates apoptosis of thymocytes and T cells, suggesting that it is a potential immunosuppressive factor (46). Conversely, extracellular galectin-3 also promotes pro-inflammatory responses that contribute to the development of autoimmune diseases (47). It has also been shown that galectin-3 induces mast cell degranulation, resulting in the release of pro-inflammatory cytokines and a prolonged inflammatory state (4849). Galectin-9 plays crucial roles in innate and adaptive immunity. In T cell immunity, galectin-9 has been demonstrated to mediate cell death and inhibit the proliferation of activated Th1 and Th17 cells (2450). Galectin-9 signaling has been found to be involved in the development of many inflammatory pathologies, including diabetes, arthritis, graft rejection, and experimental autoimmune encephalomyelitis (25,505152). These immunosuppressive properties, such as potent T cell suppressive activity and tolerance induction make galectin-9 an attractive therapeutic candidate for the treatment of several autoimmune diseases (53).
Galectin-1 and galectin-3 play important roles in MSC-mediated T cell suppression (2829). Although a recent study suggested a functional association between galectin-9 and the immunosuppressive effects of MSCs, the detailed biology of galectin-9 with regard to MSC-mediated immunosuppression has not been investigated. We hypothesized that galectin-9 could be involved in MSC-mediated immunosuppression based on its potent immunoregulatory function. We showed that galectin-9 is significantly upregulated in MSCs and secreted following stimulation with pro-inflammatory cytokines TNF-α and IFN-γ (Fig. 1). The immunosuppressive activity of MSCs is not constitutive but is induced by immune responses (1454). Thus, this inflammation induced factor could be an attractive candidate paracrine regulator. As shown in Fig. 1, galectin-9 expression was not observed in resting MSCs but was strongly induced by treatment with inflammatory cytokines. Through RNA interference-mediated knockdown experiments, we also demonstrated that induction of galectin-9 is required for MSC-mediated lymphocyte suppression (Fig. 3). Based on the comparable induction of lymphocyte apoptosisby galectin-9 and MSCs, we speculate that galectin-9 contribute to MSC-mediated immunosuppression by inducing growth arrest (Fig. 2) and T cell apoptosis (Fig. 4). Galectin-9 is a ligand of TIM-3, a type I membrane glycoprotein that is an inhibitory receptor expressed on terminally differentiated CD4 Th1 cells, Th17 cells, Tregs, natural killer cells, and dendritic cells (24555657). Galectin-9 was previously shown to induce apoptosis of TIM-3-positive Th1 cells through the interaction with TIM-3, indicating that the TIM-3/galectin-9 signaling pathway negatively regulates Th1 immune responses (2458). Our findings also revealed that rhGal-9 treatment or MSCs reduced TIM-3-positive activated lymphocytes (Fig. 4A). Considering that MSCs inhibit T cell proliferation by inducing apoptosis of activated T cells (59), we speculate that galectin-9 secreted from MSCs may could represent a principal MSC-derived factor that binds to TIM-3 and promote the internalization of galectin-9/TIM-3 (60), leading to apoptosis of activated lymphocytes (Fig. 4).
MSCs have been developed as a novel therapeutic option for several inflammatory diseases. Likewise, galectin-9 is considered to have potential to treat pathological immune disorders (6162). Our findings support the notion that galectin-9 contributes to the immunosuppressive function of MSCs. Further investigation is needed to examine how galectin-9 cooperates with other soluble paracrine factors in driving MSC-mediated immunomodulation. Further elucidation of the functional roles and mechanisms of the immunoregulatory factors produced by MSCs could provide foundation for the successful development of stem cell therapies targeting inflammatory conditions and autoimmune diseases.
Figures and Tables
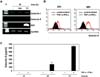 | Figure 1Galectin-9 induction in MSCs by experimental inflammatory stimulation. (A) MSCs were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ for 24 h and expression of IDO, galectin-1, galectin-9 and GAPDH was detected by RT-PCR. (B) After MSCs were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ for 24 and 48 h, flow cytometric analysis was conducted to detect intracellular expression of galectin-9. Red lines histograms indicate intracellular galectin-9 after stimulation while black-filled histograms represent unstimulated controls. (C) MSCs were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ up to 72 h and then secreted galectin-9 in the culture supernatant was quantified by ELISA. Data represent the average of three independent experiments. |
 | Figure 2The immunosuppression of galectin-9 or MSCs on human lymphocyte proliferation. (A) In vitro immunosuppressive effects of galectin-9 or MSCs on human lymphocyte proliferation were evaluated by using mitogenically activated human PBMCs (P). PBMCs stimulated with PHA (1 µg/ml) were treated with 1.5 µg/ml of recombinant human galectin-9 (rhGal-9) or co-cultured with MSCs (the cell number ratio of MSC to PBMC is 1:5) for 3 days. Lymphocyte proliferation was determined by [3H]-thymidine incorporation. (B) CFSE-labeled PBMCs stimulated with PHA were incubated with 1.5 µg/ml rhGal-9 or co-cultured with MSCs (MSC:PBMC=1:5) for 5 days followed by flow cytometric analysis for cell division. Dividing PBMCs were shown in percentage. Data shown are representative of three independent experiments. (C) Galectin-9, TNF-α, and IL-17A in the culture supernatants were quantitated by ELISA. Data are the average of three independent experiments. Statistical significance was *p<0.05 or **p<0.005. P: PBMC. |
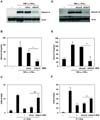 | Figure 3The effects of galectin-9-knockdown MSCs on proliferation of activated lymphocytes. (A) MSCs were transfected with siRNA oligomers specific to galectin-9 (siGal-9) or scrambled control oligomers (siCon). After the cells were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ for 48 h, western blot analysis was performed to check galectin-9 knockdown. (B) siGal-9-transfected MSCs were stimulated with10 ng/ml TNF-α and 20 ng/ml IFN-γ for 48 h and then secretion of galectin-9 was determined by ELISA. (C) PBMCs stimulated with 1 µg/ml PHA were co-cultured with siCon- or siGal-9-transfected MSCs (MSC:PBMC=1:5) for 3 days. Lymphocyte proliferation was determined by [3H]-thymidine incorporation. Galectin-9 knockdown by siGal-9-transfection abrogated the immunosuppressive activity of MSCs. Data shown are the average of three independent experiments. (D) MSCs were infected with shRNA-expressing lentivirus targeting galectin-9 (shGal-9) or control LacZ virus (shLacZ). After the cells were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ for 48 h, western blot analysis was performed to check galectin-9 knockdown. (E) The MSCs were stimulated with 10 ng/ml TNF-α and 20 ng/ml IFN-γ for 48 h and then soluble galectin-9 released into the culture supernatant was determined by ELISA. (F) PBMCs stimulated with 1 µg/ml PHA were co-cultured with shLacZ- or shGal-9-infected MSCs for 3 days. Lymphocyte proliferation was determined as in (C). MSC-suppressed proliferation of activated PBMCs was significantly recovered in the co-culture of shGal-9-transfected MSCs. Data are the average of three independent experiments. Statistical significance was *p< 0.05 or **p<0.005. P: PBMC. |
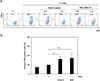 | Figure 4The effects of galectin-9 or MSCs on TIM-3-expressing lymphocytes and apoptotic analysis of activated lymphocytes by galectin-9-or MSCs. (A) After PHA-activated PBMCs were treated with exogenous rhGal-9 at a concentration of 0.3 or 1.5 µg/ml or were co-cultured with MSCs at the indicated cell ratios for 3 days, flow cytometric analysis was conducted to examine the effects of galectin-9 or MSCs on TIM-3-expressing CD3+ lymphocytes. (B) The apoptosis-inducing effects of galectin-9 or MSCs on PHA-activated PBMCs were evaluated. In the presence of rhGal-9 or MSCs, activation-induced cell death of PBMCs was significantly increased. Apoptotic cell death induced by rhGal-9 was comparable to that induced by MSCs. Apoptotic cell death was evaluated by annexin V and PI staining. Data are the average of three independent experiments. Statistical significance was *p<0.05 or **p<0.005. P: PBMC. |
ACKNOWLEDGEMENTS
This study was also supported by the Bio & Medical Technology Development Program (NRF-2011-0019634 and NRF-2014R1A2A1A11050610) of the National Research Foundation by the Korean government (MEST).
References
1. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213.

2. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.

3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.

4. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726–736.

5. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371:1579–1586.

6. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.

7. Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012; 7:e38189.

8. Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann NY Acad Sci. 2012; 1266:107–117.

9. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867.

10. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010; 28:585–596.

11. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25 (High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–160.

12. Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010; 16:203–209.

13. Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010; 316:3109–3123.

14. Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007; 149:353–363.

15. Camby I, Le MM, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006; 16:137R–157R.

16. van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008; 9:593–601.

17. Rabinovich GA, Toscano MA. Turning 'sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009; 9:338–352.

18. Matsushita N, Nishi N, Seki M, Matsumoto R, Kuwabara I, Liu FT, Hata Y, Nakamura T, Hirashima M. Requirement of divalent galactoside-binding activity of ecalectin/galectin-9 for eosinophil chemoattraction. J Biol Chem. 2000; 275:8355–8360.

19. Matsumoto R, Hirashima M, Kita H, Gleich GJ. Biological activities of ecalectin: a novel eosinophil-activating factor. J Immunol. 2002; 168:1961–1967.

20. Arikawa T, Saita N, Oomizu S, Ueno M, Matsukawa A, Katoh S, Kojim K, Nagahara K, Miyake M, Yamauchi A, Kohrogi H, Hirashima M. Galectin-9 expands immunosuppressive macrophages to ameliorate T-cell-mediated lung inflammation. Eur J Immunol. 2010; 40:548–558.

21. Irie A, Yamauchi A, Kontani K, Kihara M, Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, Hirashima M. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005; 11:2962–2968.

22. Kageshita T, Kashio Y, Yamauchi A, Seki M, Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T, Hirashima M. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002; 99:809–816.

23. Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003; 170:3631–3636.

24. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6:1245–1252.

25. Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, Nishi N, Yamauchi A, Katoh S, Matsukawa A, Kuchroo V, Hirashima M. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008; 127:78–88.

26. Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, Yamauchi A, Hattori T, Masaki T, Hirashima M. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS One. 2012; 7:e48574.

27. Sakai K, Kawata E, Ashihara E, Nakagawa Y, Yamauchi A, Yao H, Nagao R, Tanaka R, Yokota A, Takeuchi M, Hirai H, Kimura S, Hirashima M, Yoshimura N, Maekawa T. Galectin-9 ameliorates acute GVH disease through the induction of T-cell apoptosis. Eur J Immunol. 2011; 41:67–75.

28. Gieseke F, Bohringer J, Bussolari R, Dominici M, Handgretinger R, Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010; 116:3770–3779.

29. Sioud M, Mobergslien A, Boudabous A, Floisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011; 38:385–390.

30. Song SU, Kim CS, Yoon SP, Kim SK, Lee MH, Kang JS, Choi GS, Moon SH, Choi MS, Cho YK, Son BK. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008; 17:451–461.

32. Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008; 10:e17.

33. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–3843.

34. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–397.

35. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011; 6:457–478.

36. Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012; 35:213–221.

37. Dorronsoro A, Fernandez-Rueda J, Fechter K, Ferrin I, Salcedo JM, Jakobsson E, Trigueros C. Human mesenchymal stromal cell-mediated immunoregulation: mechanisms of action and clinical applications. Bone Marrow Res. 2013; 2013:203643.

38. Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011; 73:79–84.

40. Rabinovich GA, Ramhorst RE, Rubinstein N, Corigliano A, Daroqui MC, Kier-Joffe EB, Fainboim L. Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 2002; 9:661–670.

41. He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004; 279:4705–4712.

42. van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H, Zwiers P, van Weeghel R, Poppema S, Visser L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004; 204:511–518.

43. Rabinovich GA, Ariel A, Hershkoviz R, Hirabayashi J, Kasai KI, Lider O. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology. 1999; 97:100–106.

44. Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996; 93:6737–6742.

45. Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002; 21:8001–8010.

46. Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006; 176:778–789.

47. Dhirapong A, Lleo A, Leung P, Gershwin ME, Liu FT. The immunological potential of galectin-1 and -3. Autoimmun Rev. 2009; 8:360–363.

48. Frigeri LG, Zuberi RI, Liu FT. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993; 32:7644–7649.

49. Chen HY, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, Kawakami T, Hsu DK, Liu FT. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006; 177:4991–4997.

50. Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009; 39:2492–2501.

51. He W, Fang Z, Wang F, Wu K, Xu Y, Zhou H, Du D, Gao Y, Zhang WN, Niki T, Hirashima M, Yuan J, Chen ZK. Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation. 2009; 88:782–790.

52. Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006; 203:1413–1418.

53. Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009; 39:2403–2411.

54. Krampera M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011; 25:1408–1414.

55. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002; 415:536–541.

56. Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007; 81:1258–1268.

57. Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012; 119:3064–3072.

58. Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003; 4:1102–1110.

59. Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005; 19:1597–1604.

60. Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012; 91:189–196.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download