Abstract
Pulmonary edema is a major cause of mortality due to acute lung injury (ALI). The involvement of protein kinase C-δ (PKC-δ) in ALI has been a controversial topic. Here we investigated PKC-δ function in ALI using PKC-δ knockout (KO) mice and PKC inhibitors. Our results indicated that although the ability to produce proinflammatory mediators in response to LPS injury in PKC-δ KO mice was similar to that of control mice, they showed enhanced recruitment of neutrophils to the lung and more severe pulmonary edema. PKC-δ inhibition promoted barrier dysfunction in an endothelial cell layer in vitro, and administration of a PKC-δ-specific inhibitor significantly increased steady state vascular permeability. A neutrophil transmigration assay indicated that the PKC-δ inhibition increased neutrophil transmigration through an endothelial monolayer. This suggests that PKC-δ inhibition induces structural changes in endothelial cells, allowing extravasation of proteins and neutrophils.
Acute respiratory distress syndrome (ARDS) is caused by a variety of inflammatory insults, which result in acute inflammation and subsequent impairment of gas exchange and lung mechanics (1). In experimental models, ARDS is commonly called acute lung injury (ALI). Alveolar macrophages are rapidly activated in the LPS-induced ALI model and play a central role in orchestrating inflammation. They recruit neutrophils and inflammatory monocytes to the sites of pulmonary injury, a process critical for tissue damage. In particular, acute inflammation causes damage to lung epithelia and endothelia, resulting in an impaired alveolar-capillary barrier. Inflammatory mediators also increase vascular permeability. Disruption of this barrier and increased vessel permeability induce pulmonary edema.
Protein kinase C-δ (PKC-δ) belongs to the PKC subfamily and is activated by diacyglycerol (DAG) but not by calcium (2). The role of PKC-δ in ALI is controversial. An early study by Harrington's group (3) showed that PKC-δ regulates endothelial barrier function through the Rho-GTPase pathway and that PKC-δ inhibition by rottlerin results in endothelial barrier dysfunction and pulmonary edema formation. Similar functions of PKC-δ were observed in the coronary artery and brain-derived endothelial monolayers (4). However, the opposite results were reported for the function of PKC-δ in endothelial permeability (5). In this study, we revisited this issue and showed that PKC-δ regulates homeostatic endothelial permeability, as well as inflammation-induced pulmonary edema.
PKC-δ knockout (KO) mice were obtained from Dr. Keiichi I. Nakayama (6) and backcrossed to C57BL/6 mice for ten generations. Wild-type (WT) C57BL mice were obtained from Orient (Seoul, Korea). In some experiments, heterozygous littermate (PKC-δ+/-) mice were used as controls. Their response to LPS was intermediate between that of WT and PKC-δ KO mice.
Mice were anesthetized by i.p. injection of a mixture of ketamine (0.6 mg/mouse; Yuhan, Seoul, Korea) and xylazine (0.4 mg/mouse; Bayer Korea, Seoul, Korea), and LPS (60 µg in 60 µl of PBS; Sigma-Aldrich, St. Louis, MO) was administered intratracheally. Bronchoalveolar lavage (BAL) was performed using 1 ml of PBS.
Levels of IL-6, TNF-α (R&D System, Minneapolis, MN, USA), and the chemokine attractants KC, MIP-2, and MCP-1 (Peprotech, Norwood, MA, USA) in BAL fluid were determined by ELISA according to the manufacturer's instructions.
Lung tissue was fixed in 10% (v/v) formalin, embedded in paraffin, sectioned (5 µm), stained with H&E, and analyzed.
Myeloperoxidase activity was determined as previously described (78). In brief, lungs were harvested after BAL and stored at -70℃ until use. For testing, lung tissue was homogenized in 1 ml of 20 mM potassium phosphate buffer (pH 7.4) and centrifuged at 17,000 rpm for 30 min. The pellet was resuspended in 1 ml of 50 mM potassium phosphate buffer (pH 6) with 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich), sonicated, incubated at 60℃ for 2 h, and then centrifuged at 10,000 rpm for 10 min. Supernatants were then assayed in reaction buffer (530 nmol/L o-dianisidine and 150 nmol/L H2O2 in 50 mM potassium phosphate buffer), and absorbance was read at 490 nm.
For measurement of pulmonary edema, concentrations of total proteins in BAL fluid and the lung wet/dry ratio were determined. Total proteins were quantified using Micro BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Lungs were weighed immediately after harvest, and after drying at 55℃ overnight, and the wet/dry ratio was determined. In vivo vascular permeability was measured as previously described (9). In brief, Evans Blue dye (EBD; 600 µg/mouse; Sigma-Aldrich) was i.v.-injected 4 h after LPS infusion. Lungs were perfused with PBS 30 min after EBD injection, harvested, homogenized in 1 ml of formamide and incubated at 60℃ for 48 h. Supernatants were obtained via centrifugation at 10,000 rpm for 10 min and absorbance was read at 600 nm. In some experiments, cell-permeable PKC-δ activator peptideδv1-1 or inhibitor peptide ΨδRACK (0.2 µg/mouse) was intratracheally injected just before LPS infusion. Cell-permeableδv1-1 and ΨδRACK peptides (10) were synthesized by Peptron (Daejeon, Korea)
HUVEC cells were isolated as described previously (11) and low (2~3) passage cells were used for permeability and neutrophil transmigration assays. HUVEC cells (5×104 cells/plate) were seeded into 12-mm transwell plates with a 0.4-µm pore polyester membrane, and were cultured to confluence at 72 h. PKC-δ inhibitors (rottlerin: 20 µM; ΨδRACK: 1 µg/ml) were applied 30 min before treatment with LPS (1 µg/ml). For the vascular permeability assay, HRP (0.2 µg/well; Sigma-Aldrich) was added 4 h after LPS treatment. Media were harvested from the bottom well 30 min after incubation. The quantity of HRP was calculated by measuring its enzymatic activity using ABTS [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid); Sigma-Aldrich] as a substrate. The neutrophil transmigration assay was performed using a QCM Chemotaxis Assay Kit (Millipore, Billerica, MA, USA). In brief, neutrophils (2×105 cells/well) isolated from human PBMC using a standard Ficoll-Hypaque density centrifugation method were added to the upper well, and either KC (50 ng/ml) or MIP-2 (5 ng/ml) was added to the bottom well. Neutrophil migration was allowed for 15 h, and then the extent of migrating neutrophils was determined colorimetrically.
The involvement of PKC-δ in ALI is controversial. In this study, we compared the responses of WT and PKC-δ KO mice to LPS-induced ALI. To investigate the severity of pulmonary inflammation after LPS infusion, we measured levels of proinflammatory cytokines and chemokines in BAL fluid at various time points after LPS infusion. Levels of IL-6 and TNF-α in BAL fluid were markedly increased 6 h after LPS infusion and decreased to a basal level at 24 h after LPS infusion (Fig. 1A and B). Generally, these levels were not different between WT and PKC-δ KO mice at any of the time points investigated, even though levels of TNF-α were lower in PKC-δ KO mice than in WT mice 6 h after LPS infusion. Similarly, WT and PKC-δ KO mice did not show a distinguishable difference in levels of KC, MIP-2, or MCP-1 in BAL fluid (Fig. 1C~E). Overall, these results indicated that genetic deletion of PKC-δ did not show evident effects on LPS-induced pulmonary inflammation. However, histological observations revealed heavier neutrophil infiltration into the lungs of PKC-δ KO mice (Fig. 1F). In addition, there was severe perivascular edema in PKC-δ KO mice (Fig. 1F). Myeloperoxidase activity in lung tissue also indicated more rapid, heavier infiltration of neutrophils into PKC-δ KO lungs after LPS infusion (Fig. 1G). As levels of KC and MIP-2, major neutrophil chemoattractants, in PKC-δ mouse lungs were not so different from those in WT mouse lungs after the induction of ALI (Fig. 1C and D), enhanced neutrophil recruitment to the injured lungs of PKC-δ KO mice might not have been caused by dysregulation of neutrophil-chemotactic chemokine production. Moreover, PKC-δ KO neutrophils showed a similar responsiveness to KC and MIP-2 in an in vitro migration assay compared to WT neutrophils (data not shown). PKC-δ KO neutrophils also did not have any survival advantage compared to WT neutrophils when they were challenged with LPS (data not shown). Therefore, these results, along with the observation of increased pulmonary edema in PKC-δ KO mice, indicated that dysregulation of vascular permeability rather than uncontrolled inflammation is likely to be the main cause of increased neutrophil recruitment to the lungs in PKC-δ KO mice.
To test the above hypothesis, we measured the amount of total proteins in BAL fluid at various time points after LPS infusion as a parameter for protein leakage from vessels. As expected, the BAL fluid of PKC-δ KO mice contained a greater amount of total proteins at all time points investigated, relative to that of WT mice (Fig. 2A). The lung wet/dry ratio showed that the increased permeability in PKC-δ KO mice indeed resulted in severe pulmonary edema following LPS infusion (Fig. 2B). To directly show that PKC-δ KO vessels had a higher capacity for permeability, we i.v.-injected EBD 4 h after LPS infusion and measured the quantity of EBD that extravasated from vessels 30 min after EBD injection. We could easily distinguish the thicker blue lungs in PKC-δ KO mice by gross observation (Fig. 2C) and quantification of EBD extracted from lungs confirmed this result (Fig. 2C). We repeated the same EBD assay using cell-permeable PKC-δ inhibitor (10). Interestingly, in vivo injection of PKC-δ inhibitor enhanced not only steady state vascular permeability, but also LPS-initiated permeability (Fig. 2F). However, the PKC-δ activator had no effect on vascular permeability (Fig. 2F).
Finally, we explored the direct involvement of PKC-δ in permeability of an endothelial monolayer. For this, we cultured HUVEC cells in transwell plates until they reached confluence, and then performed a permeability assay. As seen in Fig. 3A, rottlerin, a purported PKC-δ inhibitor, increased permeability to a similar degree, regardless of whether or not HUVEC cells were pre-activated by LPS, indicating again that PKC-δ regulated steady state vascular permeability. Using this system, we further examined whether transendothelial migration of neutrophils could be regulated by PKC-δ. In this case, we showed that PKC-δ inhibitors (rottlerin and ΨδRACK peptide) enhanced KC- and MIP-2-induced transendothelial migration of neutrophils through the HUVEC cell monolayer only when HUVEC cells were pre-activated by LPS (Fig. 3B). These results indicated that LPS-induced upregulation of cell adhesion molecules might be required for interactions with neutrophils before their endothelial transmigration. As HEVEC cells remained healthy until the end of our experiments, it was unlikely that there was a physical lesion in the HEVEC cell monolayer.
In this study, our results suggest that PKC-δ is not involved in regulating the production of proinflammatory mediators, but that it plays a critical role in vascular permeability and transmigration of neutrophils through an endothelial cell layer. Interestingly, inhibition of PKC-δ was shown to be essential to the regulation of steady state endothelial barrier function. This regulatory function of PKC-δ is maintained during ALI. As more severe pulmonary edema occurred in PKC-δ KO mice, PKC-δ also seems to be involved in inflammation-induced vascular permeability. Even though there are contradictory reports regarding the function of PKC-δ in ALI (35), our data, obtained using a PKC-δ gene deletion mouse model and PKC-δ-specific chemical and peptide inhibitors, all support PKC-δ to be a negative regulator of vascular permeability. Further research will be needed to define the molecular mechanisms behind these observations.
Figures and Tables
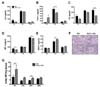 | Figure 1PKC-δ KO mice had no impairment in the production of proinflammatory mediators, but had severe neutrophil infiltration and perivascular edema. (A~E) BAL fluid was harvested at various time points after LPS infusion and levels of proinflammatory cytokines, TNF-α (A) and IL-6 (B), and chemokines, KC (C), MIP-2 (D) and MCP-1 (E), were measured by ELISA. n=4-10 for each group. *p<0.05 and ***p<0.001 between the indicated groups. (F) Lungs were harvested 12 h after LPS infusion and histological analysis was performed. Neutrophil infiltration and perivascular edema were more prominent in PKC-δ KO mice (right column). *perivascular cuff, V: blood vessel, Br: bronchus. Original magnification: ×40 (upper column) and ×200 (lower column). (G) Determination of myeloperoxidase activity in lung tissue. n=7-12 for each group. *p<0.05 and ***p<0.001 between the indicated groups. |
 | Figure 2Inhibition of PKC-δ promotes pulmonary edema and vascular permeability. (A) Total protein levels in BAL fluid at various time points after LPS infusion. n=3-14 for each group. *p<0.05, **p<0.01, and ***p<0.001 between the two groups. (B) The lung wet/dry weight ratio at 24 h after LPS infusion. n=12-13 for each group. *p<0.05. (C and D) EBD was injected 4 h after LPS infusion and lungs were harvested 30 min later. Gross observation (left column) and concentrations of EBD in extracted lung tissue (right column). n=12-13 for each group. *p<0.05. (D)δV1-1 or ΨδRACK peptide was intratracheally injected 30 min before LPS infusion. Lungs were harvested 30 min after EBD injection. n=6-8 for each group. *p<0.05 between the indicated groups. |
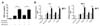 | Figure 3Inhibition of PKC-δ promotes extravasation of proteins and neutrophils through an endothelial monolayer. (A) A confluent monolayer of HUVEC cells was treated with rottlerin 30 min before the addition of LPS. Four hour later, HRP was added to HUVEC cells and extravasation was allowed to proceed for 30 min. n=3 for each group. *p<0.05 and ***p<0.001 between the indicated groups. (B) PKC-δ inhibitors and LPS were treated as described in A and neutrophil transmigration was allowed for 15 h. n=3 for each group. **p<0.01 and ***p<0.001 between the indicated groups. |
ACKNOWLEDGEMENTS
This work was funded by Ulsan University Hospital (Biomedical Research Center Promotion Fund 11-02).
References
1. Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015; 194:855–860.

2. Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003; 284:L435–L451.

3. Klinger JR, Murray JD, Casserly B, Alvarez DF, King JA, An SS, Choudhary G, Owusu-Sarfo AN, Warburton R, Harrington EO. Rottlerin causes pulmonary edema in vivo: a possible role for PKCδ. J Appl Physiol. 2007; 103:2084–2094.

4. Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, Yuan SY. Counter regulatory effects of PKCβII and PKCδ on coronary endothelial permeability. Arterioscler Thromb Vasc Biol. 2008; 28:1527–1533.

5. Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, Ayala A, Rounds S, Klinger JR, Harrington EO. Genetic disruption of protein kinase Cδ reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012; 303:L880–L888.

6. Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002; 416:865–869.

7. Lomas-Neira J, Chung CS, Grutkoski PS, Dunican A, Simms HH, Cioffi WG, Ayala A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005; 31:169–179.

8. Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, Bernacki KD, Huber-Lang M, McGuire S, Hoesel LM, Riedemann NC, Beck-Schimmer B, Zetoune FS, Ward PA. Relationship of acute lung inflammatory injury to Fas/FasL system. Am J Pathol. 2005; 166:685–694.

9. Patterson CE, Rhoades RA, Garcia JG. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J Appl Physiol (1985). 1992; 72:865–873.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download