Abstract
We previously reported that 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) accelerates hematopoiesis and has an improving effect on animal disease models such as sepsis and asthma. The effects of PLAG supplementation on immune modulation were assessed in healthy men and women. The objective was to evaluate the effects of PLAG supplementation on immune regulatory functions such as activities of immune cells and cytokine production. A randomized double blind placebo-controlled trial was conducted. Seventy-five participants were assigned to one of two groups; all participants had an appropriate number of white blood cells on the testing day. The PLAG group (n=27) received oral PLAG supplements and the control group (n=22) received oral soybean oil supplements. IL-4 and IL-6 production by peripheral blood mononuclear cells (PBMC) were lower (p<0.001 and p<0.001, respectively) with PLAG than with soybean oil. However, the production of IL-2 and IFN-γ by PBMC was unaltered with PLAG supplementation. The B cell proliferation decreased significantly in the PLAG group compared to the soybean oil control (p<0.05). The intake of PLAG in healthy adults for 4 weeks was deemed safe. These data suggest that PLAG has an immunomodulatory function that inhibits the excessive immune activity of immunological disorders such as atopic and autoimmune diseases. PLAG could improve the condition of these diseases safely as a health food supplement.
Economic growth has increased the human desire for a healthy, high-quality life,; however, chronic diseases have increased concurrently due to an aging society, environmental pollution, stress, irregular life patterns, and nutritional asymmetry. In order to prevent chronic diseases, a demand is growing for health-functional food made of natural products with fewer side effects and no toxicities. Immune function, a major biological defense system, is important in the prevention of various diseases and homeostasis of the human body. Deer horn is a popular herbal remedy that has been used in Asian cultures for thousands of years to treat a variety of conditions. It is reputed to increase resistance to stress and to enhance immune function and physical performance (12). Many studies have demonstrated that deer horn possesses anticancer, antifatigue, antibacterial, antiviral, antiinflammatory, antioxidation, and hematopoietic modulatory effects (134).
Monoacetyldiglycerides (1-palmitoyl-2-linoleoyl-3-acetylrac-glycerol [PLAG]) occur naturally in a variety of seed oils (5) and in bovine udder and milk fat (67); they have also been isolated from the horns of Sika deer (Cervus nippon Temminck) (8). PLAGs isolated from deer horns appear to demonstrate measurable biological activity, specifically stimulation of hematopoiesis in vitro (8). We have chemically synthesized PLAG from glycerol, palmitic acid, and linoleic acid, which is chemically identical to naturally derived PLAG and has been shown to stimulate proliferation of hematopoietic stem cells and bone marrow stromal cells in vitro and in vivo (48). Kim et al. have reported that PLAG has potent antitumor activity and inhibits hematogenous metastasis of biliary cancer cells in an animal model (9). Hong et al. found that PLAG markedly improves survival in a murine model of abdominal sepsis via immunomodulation (10). It was recently reported that PLAG effectively suppressed the allergic asthma response induced by ovalbumin in a murine model (11). Generally, the T-helper type 2 (Th2)-driven pheno-type arises from dysregulated innate and adaptive immune responses. IL-4 is a Th2 cytokine that plays an essential role in IgE class switching, eosinophil recruitment, mucus production and airway hyperresponsiveness (1213). IL-4/IL-4 receptor (IL-4R) pathway plays an important role in atopy and asthma (1415). However, there is no report about the immunomodulatory effects of PLAG on human immune functions until now. This clinical study showed that distinct IL-4 modulation in PLAG intake group with significance (p<0.001). This study also examines the safety and additional immunomodulatory effects of PLAG supplements in healthy adults using a randomized double-blind placebo-controlled trial.
PLAG is synthetic component which is composed of 1-palmitic acid-2-linoleic acid-3-acetylglycerol (Fig. 1), which can be separated from the chloroform extracts of the deer antler (Korean Patent No. 0283010), or alternatively can be prepared to small scale by the following 2 methods (Korean Patent Application No. 2000-0045168) and purified using several column purification steps. The first method includes the steps of obtaining reaction products by the reaction of glycerol and palmitic acid, separating 1-palmitoylglycerol by using a column chromatograpy from the reaction products, and successively carrying esterification reactions for the separated 1-palmitoylglycerol to obtain PLAG. The second method for preparing PLAG utilizes an acetolysis reaction of phosphatidyl choline. Recently, we developed the patented large scale synthetic process of PLAG without column purification. (Korean Patent No KR 10-1278874; PCT/KR2012/007644).
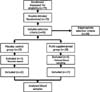
The subjects included those who 1) have body weight of ≥45 kg with BMI of 18.5~30.0 kg/m2, 2) are in subhealthy state with WBC count of 2,000~5,000 during screening, 3) can visit the hospital on schedule and make their blood taken, 4) have agreed to birth control during and after the clinical test at least for 2 month including those who have themselves sterilized, and 5) have volunteered for the research and submitted written consent to follow the clinical protocols. Those subjects were excluded from this study who 1) have troubles or histories in liver, kidney, digestive, respiratory, musculoskeletal, secretory, neurological, and cardiovascular diseases, 2) have gastrointestinal diseases (e.g. Cohn's disease and ulcer) or experienced gastrointestinal operations (excluding appendectomy and herniotomia), 3) are sensitive to medications especially those extracted from deer antlers or exhibited clinical symptoms, 4) have a history of drug addiction, 5) have unusual diet which can affect the abruption, distribution, metabolism, and excretion of the medicine within 30 days, 6) have medication for other clinical drug(s) within 60 days, 7) have donated whole blood within 60 days, or transfused within 30 days, 8) have taken a medication for the inducer or suppressor of drug metabolizing enzyme including barbitals, 9) are heavy drinkers (>21 units/week, 1 unit matches 10 ml of pure alcohol), and 10) have failed to meet the criteria of clinicians from other issues. Seventy-five subjects were enrolled from 19 November 2012 to 10 December 2012. After a sufficient explanation of the research purposes and methods, participants gave written informed consent. The study received approval from the Institutional Review Board of Myongji Hospital (IRB No. 12-083). Of the 75 subjects enrolled in the study, 24 participants were determined to be unsuitable and were excluded from the data analysis. Two subjects (one in the control group and one in the PLAG group) did not participate after 4 weeks of intervention and were excluded from the data analysis. One subject refused blood sampling, and the other travelled abroad during the study period. Baseline and post-intervention characteristics are presented for 49 subjects (22 in the control group and 27 in the PLAG group at the completion of study) (Fig. 2).
The study was designed as a 4-week double-blind, randomized, controlled trial to investigate the effect of PLAG supplements on immune function in healthy people. Subjects were divided into two groups, and doses were administered before a meal twice a day. The supplement capsule contained 500 mg PLAG and the placebo capsule contained 500 mg soybean oil (all from ENZYCHEM Lifesciences, Daejeon, Korea). The soft capsules for the PLAG supplement and the placebo were the same in color, size, and shape. Blood sampling was performed during patient fasting to measure subject's immune function prior to administering PLAG or placebo. According to the clinical protocol, subjects returned to the clinic one month after the start of administration. Doctors confirmed that the subjects had taken their soft capsules regularly and asked whether there were any side effects. On the second visit, after 4 weeks from the first visit, subjects were instructed to fast during the visit and peripheral blood samples were taken to evaluate immune function after the administration of PLAG or placebo (Fig. 2).
Peripheral blood samples were collected on two occasions: before administration and 4 weeks after administration of PLAG. All blood samples were taken after subjects had fasted for 8 hours or more. The whole blood counts including white blood cell (WBC) and red blood cell counts; leukocyte differential counts including percent neutrophils, percent lymphocytes, and percent monocytes; and hemoglobin, hematocrit, and platelet counts were surveyed with a reference automated hematology analyzer from each manufacturer. Serum biochemical values including organ function profiles such as blood urea nitrogen, creatinine, and total bilirubin; and blood metabolic profile such as blood glucose and serum enzyme profile including aspartate amino-transferase, alanine amino-transferase, and r-glutamyl transpeptidase were obtained using the span diagnostic test kit with semiautoanalyzer (CECIL Instruments Limited, Cambridge, UK) with standard techniques. Commercial kits of radial immunodiffusion plate (Biomagherb, Tunisia) were used to determine the concentrations of IgA, IgG, and IgM, and C3 and C4 complement components for the subjects. Antibodies for immunostaining flow cytometry analysis were used to measure the lymphocyte subsets, including antihuman anti-CD3-FITC (BD Biosciences, San Jose, CA, USA), anti-CD4-PE (BD Biosciences), anti-CD8-PE (BD Biosciences), and CD56-PE (BD Biosciences). In addition, 15 ml of blood samples were taken into a vacutainer tube containing heparin for ex vivo study. As such, total 15 ml of blood were diluted with the same volume of phosphate buffered saline (PBS), and then placed carefully on Ficoll-Hypaque to form layers. After centrifugation at 400 g for 30 minutes at room temperature, the buffy coat was separated and harvested and then washed twice in complete medium.
Cultured peripheral monocytes were stimulated with Concanavalin A (Con A) to secrete cytokines, followed by the centrifugal separation of supernatants. Extracted supernatants were stored at -80℃ until cytokine measurements were taken. The isolated lymphocytes were incubated with the Con A to measure IL-2 over 24 hours and to measure IFN-γ, IL-4, and IL-6 over 48 hours. The Cytokines IL-2 (Human IL-2 Quantikine ELISA kit, R&D Systems, Minneapolis, MN, USA), IL-4 (Human IL-4 Quantikine ELISA kit, R&D Systems), IL-6 (Human IL-6 Quantikine ELISA kit, R&D Systems), and IFN-γ (Human IFN-γ Quantikine ELISA kit, R&D Systems) were examined at a wavelength of 450~570 nm using an automatic microplate reader (Molecular Devices, CA, USA).
The isolated lymphocytes were tested for a proliferative response to a 48-hour challenge of the mitogens Con A and LPS. The isolated lymphocytes were plated cells in a 96-well flat-bottomed microplate after adjusting the concentration of cells to 5×104/well and incubated with the 10 µg/ml Con A or 20 µg/ml LPS to be tested at +37℃ in a humidified 5% CO2 for 48 hours. Cultured peripheral monocytes' (T cell and B cell) proliferative response to mitogens was measured with Bromodeoxyuridine (BrdU, Cell Proliferation ELISA, colorimetric). The 96-well microplate wells were labeled cells by adding BrdU and incubating the cells for 2~4 hours at 37℃ in a humidified 5% CO2. Genomic DNAs were denatured by adding Fixing Solution to each well and then added anti-BrdU antibody with peroxidase. Absorbance was measured at a wave-length of 450 nm using TMB substrate and an automatic microplate reader (Molecular Devices).
NK cell activity was measured with NKTEST® (Orpegen Pharma, Heidelberg, Germany) using fluorescence-labeled K562 target cells. Effector cells were resuspended in 1 ml of complete medium and adjusted cell concentration to 5×106 cells/ml. Fluorescently labeled K562 cells were rapidly thawed, suspended in 50 ml of prewarmed complete medium and centrifuged. Harvested cells were resuspended in 1 ml of complete medium and adjusted cell concentration to 1×105 cells/ml. Effector cells (E) were mixed with K562 target cells (T) at the desired E:T ratios ranging from 25:1 and incubated tubes for 2 to 4 hours in a humidified CO2. At the end of the incubation, tubes were placed on ice until flow cytometric analysis. Cells were analyzed with flow cytometry using the blue-green excitation light (FACSCalibur system, Becton Dickinson Biosciences, CA, USA). After the incubation period in the cytotoxicity assay, dead target cells were identified by a DNA-stain, which penetrates the dead cells and specifically stains their nuclei. This method allows the percentage of target cells killed by effector NK cells to be determined.
The phagocytic activity of leukocytes was measured using the PHAGOTEST™ (Glycotope Biotechnology GmbH, Heidelberg, Germany), which facilitates the quantitative determination of fluorescein-labelled opsonized Escherichia coli bacteria. Heparinized whole blood was incubated with the fluorescein-labelled E. coli bacteria at 37℃ and a negative control sample remains on ice. After incubation, the phagocytosis was stopped by placing the samples on ice and adding cold quenching solution. This solution enabled the discrimination between attachment and internalization of bacteria by quenching the fluorescence of surface-bound bacteria, leaving the fluorescence of internalized particles unaltered. After two washing steps with washing solution, erythrocytes are removed by the addition of Lysing solution. Cells were spun down and the supernatant discarded. After washing the samples, the DNA-staining solution was added, mixed, and put on ice prior to flow cytometric analysis, excluding aggregation artifacts of bacteria or platelets. Cells were analyzed by flow cytometry using the FACSCalibur system (Becton Dickinson Biosciences, CA, USA).
Data are expressed as the mean±SD or percentage. Sociological characteristics of the two groups were compared using an independent t-test and the Chi-square test. The paired t-test was used to compare the changes in immune function on ex vivo and peripheral blood before and after the administration of PLAG or placebo, respectively. The Mann-Whitney U test was used to calculate and compare percent change of the immune function after 4 weeks of intervention between the two groups. SPSS for Windows (Version 15.0; SPSS Inc.; Chicago, IL, USA) was used to analyze these statistics; statistical significance was defined as a p-value of less than 0.05.
Pure 1-palmitoyl-3-acetyl-rac-glycerol is a key intermediate for the preparation of pure PLAG and was synthesized using 1-palmitoyl-rac-glycerol and acetylchloride as starting materials. And resulting crude 1-palmitoyl-3-acetyl-rac-glycerol was purified in hexane to give pure 1-palmitoyl-3-acetyl-rac-glycerol. To synthesize PLAG, carboxylic acid moiety of linoleic acid was activated by pivaloylchloride in the presence of triethylamine in hexane to give corresponding mixed anhydride, and then the resulting mixed anhydride was reacted with 1-palmitoyl-3-acetyl-rac-glycerol and catalytic amount of 4-dimethylaminopyridine (DMAP) as a catalyst to give PLAG.
The two groups did not differ statistically in body mass and sex ratio of men and women (Table I). The average age of subjects in the PLAG group was 39.6 years while the average age in the placebo group was 48.8 years; incidentally, there were more older subjects in the PLAG group than in the control group (Table I). The basal status such as smoking status, alcohol intake, regular exercise, hypertension, and dyslipidemia were checked for each subject in the two groups; there was no statistical difference between the control group and the PLAG group (Table I). We checked out that there is no adverse effects on kidney or liver function in the healthy adults taking EC-18 for 4 weeks (IRB No. 12-083). Normal levels of creatinine in the blood are approximately 0.6 to 1.2 mg per deciliter (dl) in adult males and 0.5 to 1.1 mg per dl in adult females (Data not shown).
There were no statistically significant differences (p>0.05) of WBC counts before and after supplementation in both groups (Table II). Among total T cells, there was no change of CD8+ (%) T cells before and after the 4-week intervention in both the PLAG group and the control group. In the PLAG group, CD3+ (%) T cells increased from 69.8±8.4 to 72.2±7.4 and CD4+ (%) cells increased from 39.2±9.3 to 41.8±8.4. Proportion of CD56+ (NK cell, %) cells decreased from 18.5±7.9 to 16.2±7.9. In the control group, there were no differences in CD3+ (%), CD4+ (%) and CD56+ (NK cells, %) cells after the 4-week intervention. Among immunoglobulins, there was no change of IgA before or after the 4-week intervention in both the PLAG group and the control group (Table III). In the PLAG group, IgG (mg/dl) decreased from 1,327.0±170.6 to 1,240.3±184.5, and IgM (mg/dl) decreased from 114.6± 56.3 to 106.5±51.9. In the control group, IgG (mg/dl) decreased from 1,312.0±203.6 to 1,238.5±166.0, and IgM (mg/dl) decreased from 118.0±50.8 to 109.9±44.9. Among complements, C3 (mg/dl) decreased from 109.5±13.0 to 99.7±12.4 in the PLAG group and it was unchanged in the compared group. C4 (mg/dl) remained unchanged in both PLAG and control group.
NK cell activity increased from 17.1±6.5 to 22.0±4.4 in the PLAG group whereas it also increased from 15.2±6.5 to 22.2±5.2 in the control group (Table IV). B cell activity decreased from 0.27±0.48 to 0.07±0.06 in the PLAG group only (Table IV). Con A-stimulated IL-4 production (pg/ml) was well regulated from 262.1±203.1 to 98.0±56.4 in the PLAG group and from 204.8±137.8 to 116.5±65.1 in the control group (Table V). IL-6 production (pg/ml) increased from 10,427.6±7,455.3 to 5,017.1±3,692.7 in the PLAG group and from 13,862.4±15,241.8 to 7,154.8±5,847.6 in the control group (Table V). Con A-stimulated IFN-γ production was not statistically changed in both PLAG and control groups (p>0.05) (Table V).
There was no difference in percent change of phagocyte, NK cell activity, and T cell proliferation before and after 4 week intervention between PLAG and control groups (Table VI). But B cell proliferation pattern was different with statistical significance between two groups; decreased by 53.1% in PLAG group vs. by 0.2% in control group (Table VI). Among cytokine production, there was no difference in percent change of IL-2, IL-6, IFN-γ, IFN-γ/IL-4 before and after 4 week intervention between PLAG and control groups (Table VI). But IL-4 production pattern was different with statistical significance between two groups; decreased by 36.0% in control group vs. decreased by 56.1% in PLAG group (Table VI). IL-2/IL-4 production pattern was also different with statistical significance between two groups; increased by 55.1% in control group vs. increased by 173.3% in PLAG group (Table VI). C3 downregulation pattern was intend to significantly different between two groups (p=0.064, Table VI). Therefore, when we statistically re-analyzed C3 pattern after the outlier-data exclusion using the 2 Standard Deviation (SD), C3 decrease in PLAG group was statistically significant compared to the control group (p<0.05, Table VII). There was no difference in percent change of WBC, T cell, NK cell, immunoglobulins, and complements before and after 4 week intervention between PLAG and control groups (Table VI). From these data, it could be suggested that PLAG has a modulating effect on Th2 cytokine overexpression and a possible role for improvement of hypersensitive diseases (e.g., allergies, asthma, and autoimmune diseases) by controlling Th1/Th2 balance.
In this study, PLAG increased the population of T cells, especially helper T cells, which play a central role for cell-mediated immune function, particularly in the adaptive immune system. PLAG suppressed the proliferation of B cells and antibodies upon induction of excessive immune reactions using Con A in contrast to the control (soybean oil). PLAG also showed a regulatory role in Th1/Th2 balance, inhibiting the activation of Th2 by regulating secretion of IL-4. It is also suggested that PLAG has an immuno-regulatory function, inhibiting unwanted immune activations such as secretion of IL-6, an inflammatory cytokine. Given that several immunological changes with aging or westernization are a decline in T cell-mediated responses and an increase of uncontrolled frequency in immune regulation, these findings have important meanings for determining the PLAG requirements for older people or adults with immunological dysregulation.
Previous animal and human studies have shown that PLAG-stimulated hematopoiesis (48) has a potent antitumor activity (9). It has also been shown that PLAG increased the survival rate of sepsis-induced animal model via immunomodulation (10). The objective of this study was to confirm the effects of PLAG supplements on immune function of peripheral blood cells in healthy subjects and to evaluate the safety of long-term PLAG administration. It was found that the administration of PLAG for 4 weeks was safe. It was also found that the immuno-modulating effects of PLAG on the peripheral blood immune cells of healthy subjects were similar to the results of previous animal studies.
B cells are important components to humoral immunity in that they are in charge of antibody production (16). However, an excessive activation of B cells mediates chronic inflammatory reaction that causes immune diseases in the blood (16). In that respect, an immuno-modulating agent controls excessive activation of B cells. We observed that the concentration of IgG and IgM in the blood taken from the PLAG group and the control group decreased within the normal range (Table III). It is possible that PLAG may have a modulating effect for controlling excessive B cell proliferation induced by LPS and further decreases the production of immunoglobulin in the body after 4 weeks of administration.
The previous study showed that PLAG induced T cell and B cell proliferation (9). We measured the proliferation rate of T cells from blood-derived lymphocytes cultured in Con A-containing medium and did not find meaningful changes to either in the PLAG group or the control group (Table IV). This result is different from the previous in vitro studies, in which only target cells were stimulated because the human body has a variety of immune systems that interact with one another. In contrast to the control group, T cells collected from the blood of the PLAG group showed a 1.4% increase in CD3(+) total T cells and 2.7% increase of CD4(+) T helper cells, but no change in CD8(+) cytotoxic T cells (Table VI). Therefore, we hypothesize that PLAG is indirectly involved in the proliferation and differentiation of T helper cells.
To test the activity of T cells, we cultured lymphocytes stimulated with Con A, and measured the content of cytokines in the medium. Th1 and Th2 cells are classified by the type of cytokines (17181920). It is well known that Th1 cells typically produce IL-2 and IFN-γ, and Th2 cells produce IL-4, IL-5, and IL-13. IL-2 and IFN-γ are pro-inflammatory cytokines that induce phagocytes to perform phagocytosis. IL-4 and IL-13 induce antibody production of B cells by activation, and IL-5 activates eosinophils. While IFN-γ promotes the proliferation of Th1 cells, it inhibits Th2 cell proliferation. Conversely, IL-4 induces Th2 cell proliferation and inhibits Th1 cell proliferation (21). The environment of cytokines is determined by the balance between Th1 and Th2 despite a clinical controversy about the balance of Th1 and Th2, (2122). However, excessively and constitutively produced cytokines are involved in inflammatory diseases, so it is necessary to develop functional food materials for immune modulation (23). In particular, a prevalence of hypersensitive diseases continues to grow in the westernized life pattern, increasing the demand for functional foods that modulate excessively induced cytokines. Healthy foods improve immune function by normalizing the physical state of patients with immunological disorders such as allergies, asthma, or cancer, and they may reduce the risks of developing diseases in healthy individuals. In this clinical study, there was no significant change in IFN-γ and IL-2 levels before or after taking PLAG, whereas the secretion of IL-4 decreased significantly (p<0.001), compared to the soybean oil control (Table VI). In addition, the proportion of IL-2 to IL-4 and IFN-γ to IL-4 increased, suggesting that the humoral immunity switched its environment to Th1 superiority due to the decline of IL-4 secretion. Considering IL-4 is involved in particular B cells that secret IgE, IL-4 reduction in the PLAG group could be associated with the inhibition of B cell proliferation. PLAG has a modulating effect on hypersensitive diseases (e.g., allergies, asthma, and autoimmune diseases) by controlling IL-4 overexpression.
IL-6 is secreted by T cells and macrophages to stimulate immune response and has effects on the immune system, the nervous system, the endocrine system, the hematopoietic system, and the liver to maintain homeostasis. IL-6 is also involved in inflammatory and autoimmune processes in many diseases such as diabetes (24), atherosclerosis (25), Alzheimer's disease (26), systemic lupus erythematosus (27), rheumatoid arthritis (28), Sepsis (29), and prostate cancer (30). Our results showed that PLAG significantly reduced the secretion of IL-6 induced by Con A. Although it is not a significant data, it also tends that soybean oil reduced the induction of IL-6 by Con A (Table V, VI). Previous data showed that PLAG decreased the serum levels of IL-6 in a sepsis-induced mouse model and strikingly increased the survival rate compared to the control group (10). As reported, the decrease of IL-6 levels where the infection is controlled and is predictive of survival (31), IL-6 could play a major role in regulating the survival rate in sepsis-induced animals. Furthermore, IL-6 is known to be involved in the proliferation of B cells and secretion of antibodies and influences acquired immunity (29). It implies that the reduction of IL-6 in the PLAG group is associated with inhibition of B cells.
Complement has evolved as a first defense against non-self cells or unwanted host elements (32). C3 plays a central role in the activation of a complement system and contributes to innate immunity (33). Recent studies have shown that complement activation may support chronic inflammation, promote an immunosuppressive micro-environment, induce angiogenesis, and activate cancer-related signaling pathways (34). Based on these results, many studies have sought to develop a therapeutic target to treat cancer. Also complement-influencing diseases such as haemolytic uraemic syndrome (HUS), paroxysmal nocturnal haemoglobinuria (PNH), and idiopathic thrombocytopenic purpura (ITP), an autoimmune disease characterized by thrombocytopenia, are to be considered therapeutic targets; compstatin has already been developed and clinical trials for patients with these diseases are in process (35). In our study, PLAG significantly decreased serum C3 levels, but did not affect the C4 levels (Table III), suggesting that PLAG may be a good, healthy food for C3 activation-related diseases.
In the current study, we measured the activity of NK cells, the most sensitive index in the immunity test, using food and phagocytosis of granular leukocytes (23). There was no change in the number of leukocytes collected from the blood of patients and in the activity of granular leukocytes in ex vivo before and after taking PLAG. However, the group that received PLAG for 4 weeks showed more than a 40% increase in NK cell activity compared to their levels 4 weeks prior (Table VI). A similar result was observed in the control group. However, the number of NK cells with CD56 marker slightly deceased from 18.5±7.9 to 16.2±7.9% in the PLAG group; NK cell activity of the PLAG group increased when the activity rate of NK cells increased from 17.1±6.5 to 22.0±4.4. Therefore, the effect of PLAG may have a more powerful function on NK cell activity compared to the control.
Soybean oil was used as a control to PLAG in this study. Generally, soybean oil has saturated, monounsaturated, and polyunsaturated fats (36). Of the soybean oil components, linoleic acid and alpha-linolenic acid are essential fatty acids for humans. Essential fatty acids are primarily used to produce hormone-like substances that regulate a wide range of functions, including blood pressure, blood clotting, blood lipid levels, the immune response, and the inflammation response to injury infection. Considering the function of soybean oil on the immune system, it could be interpreted that several components of soybean oil might be involved in immune modulation, such as the increase of NK cell cytotoxicity, the change of serum IgG and IgM levels, and cytokine production by PBMC in control group.
In conclusion, a 4-week supplement with PLAG increased NK cell activity and T cell population, suppressed B cell proliferation, cytokine production by peripheral immune cells, and serum immunoglobulins and complements. Taken together, PLAG supplement is likely to improve the general health and provide beneficial effects in controlling several diseases including allergies, asthma, and autoimmune diseases safely.
Figures and Tables
Table I
Baseline characteristics of 75 subjects in both groups

Table II
Analysis of WBC counts, T cell subsets and NK cells in peripheral blood from PLAG and control group after 4 week intervention

Table III
The estimated levels of immunoglobulins and complements in serum from PLAG or control group after 4 week intervention

Table IV
The activity of phagocyte, T cell, B cell and NK cell from PLAG and control group after 4 week intervention

Table V
Mitogen-stimulated cytokine production of PBMCs from two groups before and after PLAG supplementation

Table VI
Comparison of the change value of the immune function between PLAG and control group

ACKNOWLEDGEMENTS
This work was supported by the KRIBB Research Initiative Program (KGM4701511) and a grant (IGM0161411) from ENZYCHEM Lifesciences.
References
1. Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013; 145:403–415.

2. Gilbey A, Perezgonzalez JD. Health benefits of deer and elk velvet antler supplements: a systematic review of randomised controlled studies. N Z Med J. 2012; 125:80–86.
3. Allen M, Oberle K, Grace M, Russell A, Adewale AJ. A randomized clinical trial of elk velvet antler in rheumatoid arthritis. Biol Res Nurs. 2008; 9:254–261.

4. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004; 27:1121–1125.

5. Kleiman R, Miller RW, Earle FR, Wolff IA. Optically active aceto-triglycerides of oil fromEuonymus verrucosus seed. Lipids. 1966; 1:286–287.

6. Myher JJ, Kuksis A, Marai L, Sandra P. Identification of the more complex triacylglycerols in bovine milk fat by gas chromatography-mass spectrometry using polar capillary columns. J Chromatogr. 1988; 452:93–118.

7. Limb JK, Kim YH, Han SY, Jhon GJ. Isolation and characterization of monoacetyldiglycerides from bovine udder. J Lipid Res. 1999; 40:2169–2176.

8. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004; 52:874–878.

9. Kim MH, Chang HM, Kim TW, Lee SK, Park JS, Kim YH, Lee TY, Jang SJ, Suh CW, Lee TS, Kim SH, Lee SG. EC-18, a synthetic monoacetyl-diacylglyceride, inhibits hematogenous metastasis of KIGB-5 biliary cancer cell in hamster model. J Korean Med Sci. 2009; 24:474–480.

10. Hong JJ, Koh Y, Park JS, Jung HD, Kim SH, Lee TS, Badellino MM. Enteral administration of a synthetic monoacetyldiglyceride improves survival in a murine model of abdominal sepsis. J Trauma. 2010; 68:62–68.

11. Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn KY, Lee TS, Kim JW, Ahn KS, Oh SR. EC-18, a synthetic monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol), attenuates the asthmatic response in an aluminum hydroxide/ovalbumin-induced model of asthma. Int Immunopharmacol. 2014; 18:116–123.

12. Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009; 31:425–437.

13. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007; 19:676–680.

14. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014; 7:53–65.
15. Isidoro-Garcia M, Davila I, Laffond E, Moreno E, Lorente F, Gonzalez-Sarmiento R. Interleukin-4 (IL4) and Interleukin-4 receptor (IL4RA) polymorphisms in asthma: a case control study. Clin Mol Allergy. 2005; 3:15.

16. Kumar VA, Abbas AK, Aster JC. Inflammation and Repair. Basic Pathology. 9th ed. Philadelphia, PA: Elsevier;2012. p. 53.
19. Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987; 138:3688–3694.
20. O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998; 8:275–283.
21. Onoe K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007; 38:319–332.

22. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003; 8:223–246.
23. Kaminogawa S, Nanno M. Modulation of Immune Functions by Foods. Evid Based Complement Alternat Med. 2004; 1:241–250.

24. Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005; 54:Suppl 2. S114–S124.
25. Dubinski A, Zdrojewicz Z. The role of interleukin-6 in development and progression of atherosclerosis. Pol Merkur Lekarski. 2007; 22:291–294.
26. Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010; 68:930–941.

27. Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004; 13:339–343.

30. Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001; 12:33–40.

31. Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 2011; 26:54–64.

32. Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009; 9:729–740.

33. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010; 11:785–797.

34. Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol. 2013; 25:54–64.

35. Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014; 123:2094–2101.

36. Hammond EG, Johnson LA, Su C, Wang T, White PJ. Soybean oil. Bailey's Industrial Oil and Fat Products. Hoboken, NJ: John Wiley & Sons, Inc.;2005. 2:p. 13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download