Abstract
Acute graft-versus-host-disease (GVHD) is characterized by selective damage to the liver, the skin, and the gastrointestinal tract. Following allogeneic hematopoietic stem cell transplantation, donor bone marrow (BM) cells repopulate the immune system of the recipient. We previously demonstrated that the acute intestinal GVHD (iGVHD) mortality rate was higher in MyD88-deficient BM recipients than that in the control BM recipients. In the present study, the role of MyD88 (expressed by donor BM) in the pathophysiology of hepatic GVHD (hGVHD) was examined. Unlike iGVHD, transplantation with MyD88-deficient T-cell depleted (TCD) BM attenuated hGVHD severity and was associated with low infiltration of T cells into the liver of the recipients. Moreover, GVHD hosts, transplanted with MyD88-deficient TCD BM, exhibited markedly reduced expansion of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSC) in the liver. Adoptive injection of the MDSC from wild type mice, but not MyD88-deficient mice, enhanced hepatic T cell infiltration in the MyD88-deficient TCD BM recipients. Pre-treatment of BM donors with LPS increased MDSC levels in the liver of allogeneic wild type BM recipients. In conclusion, hGVHD and iGVHD may occur through various mechanisms based on the presence of MyD88 in the non-T cell compartment of the allograft.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an important therapeutic modality used to treat malignancies of hematopoietic origin. Graft-versus-host disease (GVHD) is a major complication following allo-HSCT and triggers non-relapse mortality and substantial morbidity. Gastrointestinal tract damage initiated by preconditioning is a principal pathological cause of mortality from GVHD (1), exposing host epithelial and immune cells to products released by dying (eukaryotic) cells and commensal bacteria (LPS and flagellin) and thereby activating TLRs. TLRs are expressed by various cell types, including innate macrophages, dendritic cells, adaptive T and B cells (2), and non-hematopoietic epithelial cells (3). MyD88 is a primary adaptor that mediates TLR signaling and is known to deliver pro-inflammatory signals (4). However, MyD88 signaling does not always cause inflammation, as demonstrated by the incidence of more severe inflammatory bowel disease in MyD88-deficient mice than that in wild type (WT) mice (5). The recognition of pathogen-derived molecules also occurs through pattern recognition receptors including TLRs, which are widely expressed on parenchymal and nonparenchymal cell types in the liver (6).
Donor bone marrow (BM) cells repopulate the hematopoietic systems of recipients. It was previously reported that MyD88 expression in the allograft non-T cell compartment was important for protection against fatal intestinal GVHD (iGVHD) in two murine models (submitted for publication). To explore the contribution of donor BM MyD88 expression to the development of hepatic GVHD (hGVHD), we induced GVHD using T-cell-depleted (TCD) BM isolated from MyD88-deficient (MyD88KO) donor mice and T cells isolated from WT donors. Here, we observe that the MyD88-dependent expansion of CD11b+ Gr-1+myeloid derived suppressor cells (MDSC) from donor BM aggravated hGVHD severity with increasing T-cell infiltration into the liver, highlighting the importance of enhanced myeloid cell recovery from donor BM for the development of acute hGVHD.
Female C57BL/6 (B6, H-2b), B6.Ly-5a (CD45.1+), and B6D2F1 (F1, H-2b/d) mice were purchased from Japan SLC Inc. (Shizuoka, Japan). MyD88KO (B6, H-2b) mice were generated by Kawai et al. (7) and backcrossed for >10 generations onto the C57BL/6J genetic background. All animal experiments were approved by the institutional Animal Care and Use Committees of the Catholic University and Seoul National University.
To generate the MHC- and minor histocompatibility antigen-mismatched transplantation model, F1 mice pre-conditioned with total body irradiation (1,100 cGy) were i.v. injected with TCD BM cells (5×106) plus T cells (1×106 or 2×106) purified from allogeneic B6 mice or B6.Ly-5a (CD45.1+) mice, as previously described (8). For LPS pre-treatment of BM donors, LPS (2 mg/kg body weight) from Escherichia coli (O128-B12; Sigma-Aldrich, St Louis, MO, USA) was i.p. injected daily for 3 days.
Mononuclear cells were isolated from liver and peripheral lymph nodes (PLN) as previously described (9). MDSC were purified by magnetic-activated cell sorting after incubation with biotin-conjugated anti-Ly6G, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA, USA). The following antibodies against mouse antigens were purchased from BD Pharmingen (San Diego, CA, USA): FITC-conjugated anti-CD45.1 and anti-CD8; PE-conjugated anti-CD11b and anti-CD4; PerCP-Cy5.5-conjugated anti-CD8; APC-conjugated anti-Ly-6G (Gr-1); and APC-Cy7-conjugated anti-CD4. PE-conjugated anti-β 2mb Ab (B10.S) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin and eosin. Cryosections were incubated with anti-CD45.1-PE and anti-CD107b-FITC (lysosome-associated membrane protein 2, LAMP2), counterstained with DAPI (Sigma-Aldrich) following washing, and examined under a confocal microscope (LSM700; Carl Zeiss, Oberkochen, Germany).
To explore how the absence of MyD88-signaling in donor TCD BM (the non-T cell compartment) affects hGVHD progression, we induced acute GVHD in lethally irradiated F1 mice and then injected T cells purified from WT B6 mice with TCD BM isolated from MyD88KO or WT B6 mice. We have previously demonstrated that allogeneic recipients of MyD88KO TCD BM die of iGVHD earlier than WT B6 TCD BM recipients do (submitted for publication). The GVHD hosts die of severe gastrointestinal damage in this MHC- and minor histocompatibility antigen-mismatched model (1). Unexpectedly, the extent of inflammatory infiltration in the liver was less severe in the MyD88KO TCD BM recipients (Fig. 1A). These findings demonstrated that the lack of MyD88 expression in the non-T cell compartment aggravated the severity of iGVHD in association with serious intestinal damage. In contrast, the presence of MyD88 in the non-T cell compartment exacerbated the severity of hGVHD accompanied by inflammatory infiltration. Consistent with the increased hGVHD-related pathological severity, MyD88KO recipients displayed significantly lower serum ALT levels compared with those of the WT recipients (40.5±3.3 U/L vs. 81.8±10.4 U/L; p<0.01; n=3-6; Fig. 1B).
In the allogeneic recipients, irrespective of donor BM origin (i.e., MyD88KO or WT), all of the leukocytes infiltrating the GVHD target organs originated from the donor (data not shown). To more clearly compare the extent of tissue infiltration by donor T cells between the two groups of GVHD hosts, T cells isolated from congenic B6.Ly-5a B6 mice (CD45.1+) were used to induce GVHD. The proportion of CD45.1+ T cells in tissue-infiltrating leukocytes was higher in the livers of MyD88KO TCD BM recipients than that of WT TCD BM recipients, because CD45.1- non-T cell population of the former had much less cells than those of the latter (Fig. 2B). However, the absolute numbers of infiltrating CD45.1+ T cells were lower in MyD88KO TCD BM recipient livers than those of WT TCD BM recipient livers, because the total cellularity levels in the liver were lower in the former (Fig. 2A). Additionally, we quantified CD4+ and CD8+ donor T cell subtype proliferation with respect to the presence of MyD88 in this GVHD model. Differences were evident when the levels of CD8+ cell subtypes were compared with those of CD4+ cells (Fig. 2B, C). Moreover, confocal microscopic analyses revealed that CD45.1+ cells were scarcer in the liver of MyD88KO TCD BM recipients than that in WT recipient livers (Fig. 2D).
CD45.1- non-T cell compartments, which were notably rare in the MyD88KO TCD BM recipient organs analyzed (Fig. 2A), were comprised primarily of CD11b+Gr-1+ cells (Fig. 3A). The low proportion of CD11b+Gr-1+ cells in the MyD88KO group was attributed to the low levels of expansion from transplanted MyD88KO TCD BM in response to pro-inflammatory stimuli produced in the GVHD hosts. This is because the proportions of CD11b+Gr-1+ cells in naïve BM and in different peripheral organs of MyD88KO mice (i.e., prior to transplantation) were comparable to those of WT B6 mice (data not shown). However, the numbers of LAMP2+ neutrophils did not differ significantly between the two groups (Fig. 3B), indicating intact generation of neutrophils in the MyD88KO TCD BM recipients. Therefore, the reduced number of CD11b+Gr-1+ MDSC from MyD88KO BM was associated with reduced T-cell infiltration into the liver, which suggested the possibility that MDSC could enhance T cell infiltration into the liver (Fig. 2B).
Next, we explored whether supplementation of GVHD hosts with WT MDSC during the course of GVHD could compensate for the lack of MDSC expansion and increase T cell infiltration into the livers of the MyD88KO TCD BM recipients. To this end, MDSC (1×106) purified from WT or MyD88 KO BM were injected on days 3, 5, and 7 post-transplantation into GVHD hosts that had received MyD88KO TCD BM. WT MDSC supplementation increased hepatic T-cell number in the MyD88KO TCD BM recipients. In contrast, enhanced donor T cell infiltration into the liver was not observed following supplementation with MyD88KO MDSC (Fig. 4A). CD4+ and CD8+ T cell numbers were also enhanced in the livers of MyD88KO TCD BM recipients following supplementation with WT MDSC compared with those of animals supplemented with MyD88KO MDSC (Fig. 4B, C). In addition, the number of MDSC increased in the liver following WT MDSC supplementation (Fig 4D). WT MDSC supplementation into the recipients of MyD88KO TCD BM showed more efficient recruitment of donor T cells into livers than MyD88-deficient MDSCs, supporting the idea that MyD88 deficiency in donor BM not only blunted MDSC recruitment or expansion in liver, but also led to generation of MDSC functionally defective in recruiting donor T cells into the liver. These results indicated that functionally intact WT MDSC have a better capability for recruiting or expanding donor T cells than MyD88-deficient MDSC and increase hGVHD incidence.
To verify the importance of MyD88-mediated signaling by MDSC in the induction of hGVHD, we determined whether transplantation with TCD BM cells containing high levels of MDSC exacerbates hGVHD. As repeated injection of LPS elicits the development of MDSC (10), we injected BM donors with LPS prior to the preparation of TCD BM and confirmed that such pre-treatment increased MDSC frequency and Myd88 expression in the TCD BM inoculums (data not shown). Transplantation with TCD BM isolated from LPS-pre-treated donors significantly decreased the frequency of CD45.1+, CD4+, and CD8+ T cells in the livers compared with that following vehicle-pre-treated TCD BM transplantation (Fig. 5A~C). There was a trend toward an increase in the absolute number of CD45.1+, CD4+, and CD8+ T cells following transplantation with TCD BM injected LPS as compared with that after transplantation with control TCD BM that had been pre-treated with the vehicle even if the statistical significance of this increase was not observed. The proportions and number of MDSC in the livers were higher (Fig. 5D) in the test recipients than those in controls.
Following allo-HSCT, donor-derived BM cells repopulate the hematopoietic systems of recipients but may also participate in GVHD pathogenesis, because donor APC crosspresent host antigens to alloreactive donor T cells and exacerbate GVHD (11). However, with the exception of donor APC and T cells, such roles for donor-derived immune cells in hGVHD pathogenesis, have rarely been reported. In the present study, we observed that expression of MyD88 in the non-T cell component of the donor allograft (TCD BM) was important for the induction of hGVHD in a murine model. Lack of MyD88 expression in the TCD BM transplant caused incomplete expansion of MDSC and reduced donor T cell infiltration in allogeneic recipient livers. In addition, an increase in MDSC frequency and MyD88 levels in the TCD BM (achieved via pre-treatment of donors with LPS) enhanced MDSC expansion in the livers.
Inflammation is a key component of a wide range of liver diseases, including hGVHD, steatohepatitis, and acute liver failure due to systemic infections. Of the 10 human TLRs in humans, all are expressed in the liver at the mRNA level, and functional activity of most TLRs was found in the various parenchymal and nonparenchymal liver cell populations (612). It has been demonstrated that expression of the pathogen-sensing machinery in immune cells is important for liver sensitization. In chimera mice that received Propionibacterium acnes or TLR2 and TLR9 ligand treatment to induce liver sensitization, BM-derived immune cells contributed to the damage of the sensitized liver in response to endotoxin (13). In patients with hGVHD, liver biopsies reveal lymphocytic infiltration of small bile ducts with nuclear pleomorphism, epithelial cell dropout, and cholestasis in zone 3 of the liver acinus (14). Our study demonstrated that the extent of inflammatory infiltration was less severe in the MyD88KO TCD BM recipients that had inappropriate BM-derived immune cells, and the appropriate expansion of donor hematopoietic cells might be involved in the hepatic injury observed in hGVHD pathogenesis. A primary effect of MyD88-signaling in hGVHD may be aggravated inflammation via MDSC expansion, whereas the extent of MDSC expansion was reduced in MyD88-deficient mice following cecal ligation and puncture in a model of sepsis (15). In addition, MyD88-deficient mice developed more severe intestinal inflammation than the WT animals in an experimental model of inflammatory bowel disease (5), emphasizing the importance of MyD88-signaling in the protection of the host from the development of several inflammatory diseases involving the intestines.
There is a link between the immunosuppressive properties of MDSC and the extent of GVHD protection following experimental allo-HSCT (1617). MDSC, derived from embryonic stem cells (18) or generated ex vivo by IL-13 treatment (19), reduced the lethality of GVHD. Wang et al. (16), however, observed that injection of immature BM MDSC did not exert such suppressive effects. Therefore, it is probable that both pro-inflammatory and regulatory effects are exerted by MyD88-mediated signaling during the development of GVHD pathophysiology, and that these effects are cell type-specific. Thus, any protective role exerted by MyD88 may be associated with MDSC expansion in the context of GVHD, whereas the pro-inflammatory role may occur primarily in hepatic cells.
In conclusion, our results demonstrate that MyD88-dependent CD11b+Gr-1+ MDSC expansion may be associated with inflammatory cell recruitment and liver injury following transplantation. Selective MyD88 deficiency in hematopoiesis from donor TCD BM could be critical for the early induction of hGVHD following allo-HSCT, providing useful information regarding the development of target organ specificity.
Figures and Tables
 | Figure 1Allogeneic recipients of MyD88KO donor TCD-BM exhibit less severe hepatic. Lethally irradiated F1 recipients were given 5×106 WT or MyD88KO TCD-BM cells plus 1×106 purified WT T cells from allogeneic B6 donors (n=10 in each group, allo WT and allo KO). Control mice were given only TCD-BM from WT or MyD88KO mice (thus without co-transplantation of B6 T cells; n=10 in each group, WT TCD-BM and KO TCD-BM). (A) Liver histology in representative F1 recipients transplanted with WT or MyD88KO TCD-BM plus WT T cells. The extent of mononuclear cell infiltration into portal triads was assessed, and the pathological status was scored semi-quantitatively; 20 portal areas of two liver sections from each animal were evaluated. The areas within the black squares in the ×50 images of the upper panel were magnified to ×200, as shown at the bottoms of the images. The extent of pathological damage to liver was evaluated using the semi-quantitative scoring system described above, in both the allo WT and allo KO groups (n=5 in each group). (B) Serum alanine transaminase (ALT) level. Data are presented as means ± SEMs. Statistics: **p<0.01 and ***p<0.001. Data shown are representative of two independent experiments. |
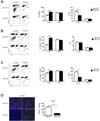 | Figure 2Effect of MyD88 deficiency in TCD-BM on the expansion and infiltration of donor-type T cells in liver and peripheral lymph nodes (PLN). Lethally irradiated F1 recipients were given 5×106 WT or MyD88KO TCD-BM cells plus 1×106 purified WT T cells isolated from congenic B6 donors (CD45.1+) (n=5, each group, allo WT and allo KO). (A) The expansion of donor T cells in the liver and PLN were assessed on day 13 post-transplantation. The frequencies and absolute numbers of CD45.1+ cells analyzed via flow cytometry are shown. Data are presented as means±SEMs and are representative of duplicate experiments. Statistics: ***p<0.001. (B and C) The frequencies and numbers of donor-type CD4+ (B) and CD8+ (C) T cells were examined on day 13. Data are presented as means±SEMs. Statistics: **p<0.01 and ***p< 0.001. (D) Infiltration of CD45.1+ cells in the liver during GVHD. Livers were harvested from F1 recipient mice (n=5) on day 13 and sectioned for staining with anti-CD45.1 Ab as described in "Materials and Methods". Magnification: ×50 (upper) and ×100 (lower). Data from one of two replicate experiments that yielded similar results are shown. |
 | Figure 3Effect of MyD88 deficiency in TCD-BM on the expansion of CD11b+Gr-1+ MDSC. Lethally irradiated F1 recipients were given 5×106 WT or MyD88KO TCD-BM cells plus 1×106 purified WT T cells isolated from WT B6 donors (n=5, each group, allo WT and allo KO). (A) On day 13 thereafter, the liver and peripheral lymph nodes (PLN) were harvested and the expression levels of CD11b+Gr-1+ were measured via flow cytometric analysis. The proportions and absolute numbers of CD11b+Gr-1+ cells are shown. Data are presented as means ± SEMs and are representative of duplicate experiments. Statistics: ***p<0.001. (B) Peripheral blood mononuclear cells (PBMC) from GVHD hosts that received WT or MyD88KO TCD-BM (n=3 in each group, allo WT and allo KO, respectively) were stained with anti-LAMP2 antibody. The numbers of LAMP2-positive neutrophils in immunofluorescence fields were compared between the allo WT and allo KO groups. The data shown are representative of two independent experiments. |
 | Figure 4Injection of CD11b+Gr-1+ MDSC from WT mice, but not MyD88KO mice, enhances donor T-cell infiltration into the livers. Lethally irradiated F1 recipients were given 5×106 MyD88KO TCD-BM cells plus 1×106 purified WT T cells from allogeneic B6 donors. Mice exhibiting GVHD that had received MyD88KO TCD-BM cells were injected with 1.0×106 CD11b+Gr-1+ MDSC isolated from WT or MyD88KO mice at days 3, 5, and 7 post-transplantation. (A~D) The frequencies and absolute numbers of donor T cells (A) with donor-type CD4+ (B) and CD8+ (C) T cells analyzed via flow cytometry are shown. (D) Expression of CD11b+Gr-1+ was measured using FACS analysis. The frequencies and absolute numbers of CD11b+Gr-1+ cells are shown. Data are presented as means±SEMs. Statistics: *p<0.05, **p<0.01 and ***p<0.001. Data are representative of duplicate experiments (n=6 per experiment). |
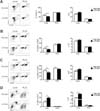 | Figure 5Recipients of TCD BM from WT donor mice injected with LPS show high MDSC levels in the liver with decreased frequency of donor T cells. WT or MyD88KO B6 donor mice were subjected to i.p. injection of LPS at 2 mg/kg for 3 days. Mice not receiving LPS received an equal amount of the LPS vehicle (PBS i.p.). Lethally irradiated F1 recipients were given 5×106 LPS- or PBS-treated donor TCD-BM cells plus 1×106 purified WT T cells from allogeneic B6 donors (n=6 per group). (A~D) The frequencies and absolute numbers of donor T cells (A), and donor-type CD4+ (B) and CD8+ (C) T cells analyzed via flow cytometry are shown. (D) The expression levels of CD11b+Gr-1+ were measured via FACS. The frequencies and absolute numbers of CD11b+Gr-1+ cells are shown. The data are presented as means±SEMs. Statistics: **p<0.01 and ***p<0.001. Data are representative of duplicate experiments. |
ACKNOWLEDGEMENTS
This study was supported by the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare, and Family Affairs, Republic of Korea (Grant no. A120262).
References
1. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213.

2. Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005; 175:1551–1557.

3. Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Gobel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010; 59:1079–1087.

4. Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009; 113:2256–2264.

5. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118:229–241.

6. Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology. 2006; 44:287–298.

7. Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999; 11:115–122.

8. Min CK, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara JL, Reddy P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004; 104:3393–3399.

9. Comer GM, Ramey WG, Kotler DP, Holt PR. Isolation of intestinal mononuclear cells from colonoscopic biopsies for immunofluorescence analysis by flow cytometry. Dig Dis Sci. 1986; 31:151–156.

10. De Wilde V, Van RN, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, Cuturi MC, Goldman M, Le MA. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009; 9:2034–2047.

11. Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004; 10:987–992.

12. Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002; 168:554–561.

13. Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, Szabo G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008; 48:1342–1347.

14. Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB. A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology. 1988; 8:463–470.

15. Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007; 204:1463–1474.

16. Wang D, Yu Y, Haarberg K, Fu J, Kaosaard K, Nagaraj S, Anasetti C, Gabrilovich D, Yu XZ. Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant. 2013; 19:692–702.

17. Ghansah T, Paraiso KH, Highfill S, Desponts C, May S, McIntosh JK, Wang JW, Ninos J, Brayer J, Cheng F, Sotomayor E, Kerr WG. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J Immunol. 2004; 173:7324–7330.

18. Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010; 28:620–632.

19. Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010; 116:5738–5747.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download