Abstract
Controlling balance between T-helper type 1 (Th1) and T-helper type 2 (Th2) plays a pivotal role in maintaining the biological rhythm of Th1/Th2 and circumventing diseases caused by Th1/Th2 imbalance. Interleukin 4 (IL-4) is a Th2-type cytokine and often associated with hypersensitivity-related diseases such as atopic dermatitis and allergies when overexpressed. In this study, we have tried to elucidate the function of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) as an essential modulator of Th1/Th2 balance. EC-18 has showed an inhibitory effect on the production of IL-4 in a dose-dependent manner. RT-PCR analysis has proved EC-18 affect the transcription of IL-4. By analyzing the phosphorylation status of Signal transducer and activator of transcription 6 (STAT6), which is a transcriptional activator of IL-4 expression, we discovered that EC-18 induced the decrease of STAT6 activity in several stimulated cell lines, which was also showed in STAT6 reporter analysis. Co-treatment of EC-18 significantly weakened atopy-like phenotypes in mice treated with an allergen. Collectively, our results suggest that EC-18 is a potent Th2 modulating factor by regulating the transcription of IL-4 via STAT6 modulation, and could be developed for immune-modulatory therapeutics.
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) occurs naturally in a variety of seed oils (1) and in bovine udder and milk fat (2,3); it has also been isolated from the horns of the Sika deer (Cervus nippon Temminck) (4). EC-18 that has been isolated from deer horns has demonstrated measurable biological activity, specifically the stimulation of hematopoiesis in vitro (4). Previously, we have chemically synthesized EC-18 from glycerol, palmitic acid, and linoleic acid; this form of EC-18 is chemically identical to naturally derived EC-18 (5,6) and has been shown to stimulate the proliferation of hematopoietic stem cells and bone marrow stromal cells in vitro and in vivo (4,7). Kim et al. reported that EC-18 had potent antitumor activity and inhibited the hematogenous metastasis of biliary cancer cells in an animal model (8). Hong et al. found that EC-18 markedly improved survival in a murine model of abdominal sepsis via immunomodulation (9). It was recently reported that EC-18 effectively suppressed the allergic asthma response induced by ovalbumin in a murine model (10). Therefore, EC-18 is considered to be one of the agents with biological activity, such as cytokine modulation (9), anti-metastatic effects (8), and hematopoiesis (7). However, the intracellular working mechanism of EC-18 has not been well verified. In this study, we have tried to shed light on the role of EC-18 in modulating the signal molecules associated with T-helper type 2 (Th2)-related immune regulation, especially the regulation of interleukin 4 (IL-4) expression.
IL-4 has been intensively studied and is still one of the most interesting and biologically significant cytokines (11). IL-4 is mainly expressed in Th2 cells and has diverse biological activities (12). Recent studies have shown an enhanced expression of the IL-4 cytokine in the region with hypersensitive immune responses, such as atopic dermatitis (13,14,15,16), asthma (17,18,19), and allergies (20,21). An imbalance between Th1 and Th2 was easily detected in these diseases; the most commonly observed phenomenon was enhanced Th2-type cytokines like IL-4 and IL-13. The signal transducer and activator of transcription 6 (STAT6) is required for mediating responses to IL-4 and for the development of Th2 cells (22). Interferon-gamma (IFN-γ) is the main cytokine released from Th1 cells and also controls the differentiation of na?ve CD4 T cells into CD4+ effector T cells, which promotes cell-mediated immune responses and is required for host defense against intracellular viral and bacterial pathogens (23). Canonically, the IFN-γ/JAK/STAT1 signaling pathway is well known (24). Therefore, it was assumed that IFN-γ expression and STAT1 activation are a major response of a Th1-biased immune response.
In this study, we focused on EC-18's ability to modulate the Th1/Th2 equilibrium. The phosphorylation and transcriptional activity of STAT1/STAT6 and the expression of IFN-γ /IL-4 via treatment with EC-18 were investigated in in vitro and in vivo assays. In particular, the possibility of EC-18 having a therapeutic effect on Th2-associated diseases was tested via its ability to downregulate IL-4 and dephosphorylate STAT6.
EC-18 is synthetic component that is composed of 1-palmitic acid-2-linoleic acid-3-acetylglycerol (Fig. 1). In this study, EC-18 was synthesized using a patented process (Korean Patent Application No. 10-2005-0065792) from 1-palmitoylrac-glycerol as a starting material (5). We recently developed a patented method for the large-scale synthesis of EC-18 without column purification (Korean Patent No KR 10-1278874; PCT/KR2012/007644).
The A549, HEK293, Jurkat, NK-92, RBL-2H3, and U937 cell lines were purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA). The cells were cultured at 37℃ in a 5% CO2 humidified atmosphere. The A549 and HEK293 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; GIBCO-BRL, Invitrogen, Carlsbad, CA, USA). The Jurkat and U937 cells were cultured in the RPMI-1640 medium (GIBCO-BRL) supplemented with 2 mM glutamine and 10% fetal bovine serum (FBS; Hyclon, Logan, UT, USA). The IL-2-dependent NK-92 cells (human NK lymphoma) were maintained in α-MEM (Life Technologies, Karlsruhe, Germany) containing 20% FCS (HyClone), 2 mM L-glutamate, 100 µg/ml penicillin, and 100 µg/ml streptomycin (Life Technologies) and supplemented with 100 U/ml IL-2 (Chiron, Emeryville, CA, USA).
A chemical inhibitor of STAT1 (Fludarabine) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), a STAT6 inhibitor (AS1517499) was purchased from Axon Medchem BV (Groningen, The Netherlands), and human recombinant IL-4 and IFN-γ were obtained from BioSource (Camarillo, CA, USA). Phorbol 12-myristate 13-acetate (PMA) and dinitrophenyl-human serum albumin (DNP-HSA) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
RBL-2H3 and NK-92 cells were seeded in a 48-well plate at 2×105 cells/ml. The RBL-2H3 cell was pretreated with anti-DNP-IgE (50 ng/ml) for 12 h, stimulated with DNP-HAS (25 ng/ml) for 2 h, and then treated with EC-18 (0.001-10 µg/ml) for 2 h. On the other hand, the NK-92 cell was activated with PMA (500 ng/ml) for 24 h and then treated with EC-18 (0.001 -10 µg/ml) for 2 h before the cells were harvested. Total cellular RNA was extracted using the TRIzol reagent (Invitrogen). The cDNAs were synthesized from total cellular RNA using oligo-dT primers (Promega, Madison, WI, USA) with the Moloney murine leukemia virus reverse transcriptase (M-MLV RT, Promega) at 42℃ for 1 h following the manufacturer's instructions. One microliter of the synthesized cDNA was used per 20 µl PCR reaction, which was comprised of 0.5 U ExTaq DNA polymerase, 1×buffer, and 1 mM dNTP mix (Takara Korea Biomedical, Inc., Seoul, Korea) with specific primer pairs. It was amplified as follows: 94℃ for 5 min; then 25 to 40 cycles at 94℃ for 45 s, 56℃ for 45 s, and 72℃ for 1 min; followed by a final extension of 7 min at 72℃ using the GeneAmp PCR system 2700 (Applied Biosystems, Foster City, CA, USA). The PCR primers were designed using the Primer3 program and were purchased from Bioneer (Daejeon, Korea). The PCR products were separated in 1.5% agarose gel, stained with ethidium bromide, visualized using the Gel Doc 2000 UV trans-illuminator (Bio-Rad Laboratories, Hercules, CA, USA), and analyzed using Quantity One software (Bio-Rad Laboratories). Each sample was tested more than three times, and representative data are shown. For IL-4, we used the primer pairs of 5'-AATGGGTCTCACCTCCCAAC-3' (F) and 5'-TTCAGCTCGAACACTTTGAA-3' (R); for IFN-γ, we used the primer pairs of 5'-TGGCTGAACTGTCGCCAGCA-3' (F) and 5'-TGGCTGCCTAGTTGGCCCCT-3' (R); and for GAPDH, we used the primer pairs of 5'-CCATCACCATCTTCCAGGAG-3' (F) and 5'-ACAGTCTTCTGGGTGGCAGT-3' (R).
We seeded RBL-2H3 and NK-92 cells in a 48-well plate at 2×105 cells/ml. The RBL-2H3 cells were pretreated with anti-DNP-IgE (50 ng/ml) for 12 h, stimulated with DNP-HAS (20 ng/ml) for 6 h, and then treated with EC-18 (0.001-10 µg/ml) for 2 h. On the other hand, the NK-92 cells were activated with PMA (500 ng/ml) for 24 h and then treated with EC-18 (0.001-10µg/ml) for 2 h before the cells were harvested. The inhibitory effects of STAT1 inhibitor (Fludarabine) and STAT6 inhibitor (AS 1517499) on IFN-γ and IL-4 production were confirmed in NK-92 and RBL-2H3 cells, respectively. The levels of cytokine secretion in the cell supernatants or plasma were analyzed using an enzyme- linked immunosorbent assay (ELISA) set specifically for cytokines from BD Bioscience (San Diego, CA, USA) according to the manufacturer's protocol. The absorbance was measured at 450 nm using an EMax Endpoint ELISA microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA) and then recalculated as a concentration (pg/ml) using a standard curve with the SOFTmax curve-fitting program (Molecular Devices Corporation).
The cells were washed with phosphate-buffered saline (PBS) and lysed with cell lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM NaF, and Complete Protease Inhibitor Cocktail; Roche, Indianapolis, IN, USA) on ice for 30 min. Thirty to fifty micrograms of the cell lysate was resolved by SDS-PAGE on 10% or 12% gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were incubated with primary antibodies followed by peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibodies (Calbiochem, EMD Chemicals Inc., San Diego, CA, USA) and the ECL reagent (Millipore) for band visualization. To verify equal loading and adequate transfer, the membranes were probed with anti-α -GAPDH antibodies (Santa Cruz Biotechnology). The primary antibodies were anti-STAT1, anti-phospho-STAT1, anti-STAT6, and anti-phospho-STAT6 (Cell Signaling Technology, Beverly, MA, USA).
Cells at 40% to 50% confluence in the 12-well plates were transfected with a reporter luciferase plasmid containing the interferon stimulated response element (ISRE) (pGL4.45[luc2P/ISRE/Hygro]; Promega) and containing four tandem copies of the STAT6 binding site (p4xSTAT6- Luc2P; Addgene, Cambridge, MA, USA). The reporter vector was then transfected into the cell lines with Lipofectamine plus reagent (Lifetechnology) according to the manufacturer's instructions. The total amount of plasmid DNA per well was adjusted to be the same by adding suitable amounts of empty vector. To activate the STAT1 and STAT6 reporter vectors, IL-4 (10 ng/ml) and IFN-γ (10 ng/ml) were used to treat to the cells for 6 h. Cells were harvested 48 h after transfection, and luciferase activity was measured using a commercial luciferase assay kit (Promega) on the TD20/20 Turner luminometer (Turner Design Instruments, Sunnyvale, CA, USA). The luciferase activity of each sample was normalized to that of the corresponding sample transfected with pGL4. All experimental and control groups contained at least three wells, and the results were reported as mean absorption±standard error.
Six-week-old male BALB/c mice were purchased from CoreTech (Pyeongtaek, South Korea) and randomly assigned to one of two experimental groups (n=4 mice/group). The groups were either treated with EC-18 or olive oil. The animals were maintained under specific pathogen-free conditions and used for the experiment after 7 days of acclimation. Experimental procedures were carried out in accordance with guidelines and under the approval of the Institutional Review Committee for Animal Care and Use of the Korea Research Institute of Bioscience and Biotechnology (KRIBB-AEC-13107). The mice were anesthetized, and their backs were shaved. They were then exposed to dinitro-1-chlorobenzene (DNCB; Sigma-Aldrich). The chemicals were dissolved in a vehicle with a 1:4 ratio of ethyl acetate to olive oil. The mice were sensitized with 0.2% DNCB 6 times and with 1% DNCB on last time over 7 days and then challenged with 0.2% DNCB every 2 days for a total of 5 times. EC-18 was dissolved in olive oil at a concentration of 2 mg/100 µl and orally administrated at a dose of 100 mg/kg body weight every day for 18 days. Blood samples were collected on Day 0, Day 4, Day 14, and Day 18 from each mouse by eye bleeding. Plasma was prepared by centrifugation and stored at -80℃ for the ELISA assay.
We first tried to elucidate the function of EC-18 for controlling the Th1/Th2 balance in immune cells. Therefore, we checked the modulation of IL-4 and IFN-γ expression in EC-18-treated cells using RT-PCR (Fig. 2) and ELISA (Fig. 3). The transcript for IL-4 was induced via FcεR1 stimulation of the mast cell, RBL-2H3, as shown in Fig. 2A. DNP-HSA antigen (25 ng/ml) -stimulated RBL-2H3 cells showed an effective IL-4 mRNA expression within 2 h of treatment. EC-18 significantly interrupted an induction of IL-4 mRNA transcripts by antigen in a dose-dependent manner (Fig. 2A). On the other hand, the PMA-induced IFN-γ expression in NK-92 cells was not affected by EC-18 treatment in a dose-dependent manner (Fig. 2C). We also found that antigen-induced IL-4 transcripts were decreased by the STAT6 inhibitor (AS 1517499) in RBL-2H3 cells (Fig. 2B) and that the STAT1 inhibitor (Fludarabine) was capable of reducing PMA-modulating IFN-γ expression in NK-92 cells (Fig. 2D). These results show that EC-18 has an effect on modulating of IL-4 expression dependent on STAT6 activation but has no effect on IFN-γ expression related to STAT1 activation.
As a biological process, IL-4 secretion was evaluated via the ELISA system in the IgE-stimulated RBL-2H3 mast cells. Anti-DNP-IgE-treated cells were stimulated by DNP-HAS (25 ng/ml) for 6 h and treated with 10, 1, 0.1, 0.01, and 0,001 µg/ml of EC-18 for 2 h. After being cultured, secreted IL-4 in the culture supernatant was calculated using the ELISA kit. As shown in Fig. 3A, secreted IL-4 by antigen stimulation was effectively blocked by EC-18 treatment (p<0.001). However, EC-18 was unable to inhibit IFN-γ production in PMA-stimulated NK-92 cells as shown in Fig. 3C. The STAT6 inhibitor (AS 1517499) was used as a relevant control for the downregulation of IL-4 secretion from the cells. As shown in Fig. 3B, IL-4 expression was apparently blocked by the dose-dependent manner of the STAT6 inhibitor. On the other hand, the STAT1 inhibitor had a distinct role in the suppression of IFN-γ expression in a dose-dependent manner (Fig. 3D). These results confirm that EC-18 has a modulating effect on the downregulation of IL-4 expression and protein secretion that is associated with Th2-related immune regulation. This suggests that the functional role of EC-18 as an IL-4 regulator might be utilized for recovering from the alternatively activated immune microenvironments.
It is well known that STAT6 activation is generally mediated by IL-4 receptor stimulation. Activated STAT6 has been found to translocate from the cytoplasm to the nucleus and to induce target genes including IL-4, IL-5, and IL-13 (25). To determine whether EC-18 directly modulates activated STAT6 phosphorylation, we investigated the effect of EC-18 on the modulation of IL-4 induced STAT6 phosphorylation. The treatment of 10 ng/ml of IL-4 activated STAT6 in U937 (a human monoblast cell line), A549 (a human lung carcinoma epithelial cell line), and Jurkat (a human T lymphocyte cell line). IL-4 induced phosphorylation of STAT6 was effectively blocked in the EC-18-treated cells in a dose-dependent manner, as shown in Fig. 4A, 4C, and 4D. The quantity of loaded protein was normalized with the STAT6 protein. As a comparative control, the phosphorylation of STAT1 was examined in the EC-18-treated cells' lysate with EC-18 concentrations ranging from 0.01 to 10 µg/ml. Phosphorylation of STAT1 was induced by 10 ng of IFN-γ treatment. As shown in Figure 4B, dephosphorylation of STAT1 was not found in the EC-18-treated cells. These data show that EC-18 has a potent ability to block the phosphorylation of STAT6 but not STAT1.
Next, we confirmed whether EC-18 modulates the transcriptional activity of STAT6 by promoter analysis. A STAT6 reporter gene, pGL4-STAT6-Luc-hygro vector, containing STAT6-binding DNA elements was transfected into HEK293 and A549. The EC-18-treated cells showed that STAT6 transcriptional activity was significantly reduced in a dose-dependent manner as shown in Fig. 4A and 4C. An inhibitory effect of STAT6 transcriptional activity by EC-18 was more sensitive in the A549 cells. As a comparative group, pGL4-STAT1-Luc-hygro vector was also transfected into HEK293 and A549 using the same protocols as those of STAT6. STAT1 transcriptional activity was induced by 10 ng/ml of IFN-γ treatment. As shown in Figures 4B and 4D, EC-18 did not have an inhibitory effect on STAT1 transcriptional activity in the HEK293 and A549 cells. Also, we checked that STAT5 dephosphorylation was not affected by EC-18 (these data are not shown). These data suggest that EC-18 has an effect on the inhibition of STAT6 transcriptional activity with specificity.
To determine whether EC-18 affects the modulation of signal molecules associated with the Th2-related immune regulation, such as the regulation of IL-4 plasma levels, 100 mg/kg EC-18 was orally administrated to DNCB-induced atopy-like dermatitis mice every day for 18 days. The plasma levels of IL-4 and IgE were significantly reduced in the EC-18 treated group at 3 weeks after DNCB sensitization compared with the control group (Fig. 6A). Plasma levels of total IgE in the DNCB-challenged mice were markedly elevated at 3 weeks in both the EC-18 treated and control groups. However, the EC-18 treated group showed a significant reduction in their plasma IgE levels compared to the control group (Fig. 6B). When we visually observed the skin condition of the DNCB-challenged mice, the EC-18 diet was found to have an effect on improving DNCB-induced dermatitis compared with the control (Fig. 6C). These results suggest that EC-18 has a possible role as an IL-4 regulator, which could be utilized in recovering from alternatively activated immune microenvironments.
Deer antler, from which EC-18 has been isolated, is a major component of Asian traditional medicine. It has been used as a biological activator and dieted preferentially as a valid remedy over 20 centuries (26,27). EC-18 has been identified as a main component of an extract that has a biological effect on immune modulation (4,7,9,10), and it has been synthesized as a compound substance to produce new drug (5,6). In this study, the scientific evidence for evaluating EC-18's efficacy in immune modulation was considered.
In this study, EC-18 has been found to effectively block STAT6 activation through its dephosphorylation with specificity (Fig. 4). STAT1 activation induced by its phosphorylation was observed in the IFN-γ-treated U937 cells; we also found that treatment with EC-18 does not disturb STAT1 phosphorylation (Fig. 4 and 5). The role of EC-18 in inhibiting STAT6 activated with IL-4 means that it has a target molecule that is associated with STAT6 inactivation. Therefore, selective blocking of EC-18 against IL-4 signaling has a meaningful biological significance for controlling the immune response toward Th1 polarity. EC-18 could serve as one of several prominent extrinsic factors that alter the immune response and lead to a recovery from degenerative diseases; for example, it could be used as a therapeutic tool for atopic dermatitis and for the improvement of tumor microenvironments.
In a previous report, we showed that EC-18 significantly suppressed the allergic asthma response induced by aluminum hydroxide/ovalbumin in mice and also reduced elevated Th2 cytokines, IgE, IgG, and eotaxin-1 levels (10). It is well known Th2 cytokines, such as IL-4, IL-5, and IL-13, play an important role in the initiation and progression of atopic dermatitis (28). Of the Th2 cytokines, IL-4 induces B-cell activation and differentiation into immunoglobulin E (IgE)-producing plasma cells (29) and subsequently activates mast cells with the high-affinity IgE receptor (FcεR1). The activated mast cell undergoes degranulation and releases the mediator for the recruitment of immune cells (30). Similar to previous data, we have found that EC-18 effectively reduces overexpressed IL-4 mRNA and protein in several immune cell lines (Fig. 2 and 3). However, to date, we do not have finalized data related to the functional role that EC-18 may play in Th1/Th2 balance and the intracellular signaling mechanism of EC-18.
We observed IL-4 downregulation by EC-18 treatment through an analysis of reduced mRNA transcripts and secreted IL-4, which convincingly demonstrated that EC-18 suppresses IL-4 expression via its inhibition of the STAT6 signaling pathway (Fig. 2B and 3B). Inactivation of STAT6 transcriptional activity by its dephosphorylation subsequently resulted in reduced IL-4 transcripts. In line with the reduced mRNA of IL-4, a diminished IL-4 secretion was verified by ELISA. It is well known that, IL-4 overexpression skews the immune response toward Th2 (31,32), which means that the arbitrary regulation of exaggerated IL-4 using extrinsic factors could be an effective tool for recovery from Th2-driven diseases. Phosphorylation of STAT6 is generally mediated by IL-4 receptor stimulation. Activated STAT6 translocates from the cytoplasm to the nucleus and induce target genes, including IL-4, IL-5, and IL-13 (25). SOCS proteins have been found to negatively regulate STAT6 activation and reduce the susceptibility of allergies and asthma, and a deficiency to these proteins enhanced differentiation into the Th2 of helper T cells (33,34). M2 or M2-like macrophage polarization is facilitated by cytokines that signal via STAT3 and STAT6, such as IL-10 or IL-4 and IL-13 (35). Modification of STAT6 activity has distinct biological activity in the activation of humoral immunity, and STAT6 activation is pivotal for the generation of IL-4 transcripts. Inhibiting STAT6 phosphorylation is a potent approach for attenuating IL-4 expression. Effective interruption of STAT6 phosphorylation could be useful in skewing the immune response from Th2 to Th1. In this paper, we reveal that EC-18 is a potential converter from Th2 to Th1 via the effective modulation of IL-4 cytokines by blocking STAT6.
IFN-γ controls the differentiation of naive CD4 T cells into Th1 effectors, which mediate cellular immunity against viral and intracellular bacterial infections (23). IFN is a typical Th1 cytokine, and its expression effects on Th2-type cytokines like IL-4 and IL-13. IL-4 and IFN-γ do not directly inhibit differentiated Th1 or Th2 cells, instead, they are inhibitory by blocking the differentiation of those subsets from naive precursors (36). IFN deficiency has been observed in asthmatic patients and was associated with airway hyperresponsiveness (37). In addition, total serum IgE levels have been found to correlate negatively with IFN levels (38). These findings suggest that Th1 polarity and a high level of IFN-γ has potential as a promising therapy for atopy and asthma (39). Canonical IFN-γ-JAK-STAT1 signaling is a well-known pathway for transcriptional activity of STAT1 (24). Ligand engagement of the IFN-γ receptor leads to activation of Jak1 and Jak2 phosphorylation and leads to the phosphorylation of STAT1. Thus, IFN-γ expression and STAT1 phosphorylation are a typical phenomenon of a Th1-biased immune response. In the current study, we found that the modulation of inducible IFN-γ expression and STAT1 activation was not changed by EC-18 (Fig. 2C, 3C, 4B, 5B, and 5D).
Therefore, when taken together, the verified EC-18 efficacy in the modulation of immune balance could be utilized as a therapeutic tool for hypersensitivity-related diseases, such as allergies and atopic dermatitis.
Figures and Tables
 | Figure 2EC-18 reduced antigeninduced IL-4 mRNA transcripts in RBL-2H3 cells. RBL-2H3 cells were treated with anti-DNP-IgE (50 ng/ml) for 12 h followed by stimulation with DNP-HSA antigen (25 ng/ml) for 2 h, and NK-92 cells were treated with PMA (10 ng/ml) for 24 h, respectively. EC-18 was pretreated from 0.01 to 10 µg/ml both in RBL-2H3 (A) and NK-92 (C). As positive controls, STAT6 inhibitor (AS 1517499) and STAT1 inhibitor (Fludarabine) was pretreated in RBL-2H3 (B) and NK-92 (D), respectively. After incubation for 2 h, mRNA was isolated from each group of cells and RT-PCR was carried out. As an internal control, GAPDH was used. |
 | Figure 3EC-18 decreased the levels of IL-4. Secreted IL-4 and IFN-γ were evaluated using ELISA. IL-4 and IFN-γ were produced using the DNP-HSA antigen (25 ng/ml) and PMA (10 ng/ml) in RBL-2H3 (A, B) and NK-92 (C, D) cells, respectively. EC-18 was pretreated from 0.001 to 10 µg/ml in RBL-2H3 (A) and from 0.001 to 10 µg/ml in NK-92 (C). As positive controls, the STAT6 inhibitor (AS 1517499) and STAT1 inhibitor (Fludarabine) were pretreated in RBL-2H3 (B) and NK-92 (D), respectively. After 12 hours of cell culture, culture supernatants were harvested and the produced IL-4 and IFN-γ were quantitated by ELISA. Each bar represents the mean±SD. Significance (p<0.001), indicated by **, is the EC-18-treated cells versus the antigen only-treated cells. |
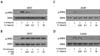 | Figure 4EC-18 dephosphorylated activated STAT6. Phosphorylation of STAT6 was examined using antiphospho STAT6 in the U937 (A), A549 (C), and Jurkat (D) cell lysates treated with EC-18 with concentrations from 0.01 to 10 µg/ml. Phosphorylation of STAT6 was induced by 10 ng/ml of the IL-4 treatment. (B) STAT1 phosphorylation was checked out in the cell lysate treated EC-18 with concentrations from 0.01 to 10 µg/ml. The activation of STAT1 was induced by 10 ng of the IFN-γ treatment in the U937 cells. Dephosphorylation of STAT1 and STAT6 was examined at 15 min after stimulation with IFN-γ and IL-4, respectively, in the EC-18 pretreated cells. |
 | Figure 5EC-18 inhibited the transcriptional activity for STAT6, but not for STAT1. One µg of pGL4- STAT6-Luc-hygro or pGL4-STAT1- Luc-hygro vector was transfected into HEK293 (A, B) and A549 (C, D) cells, and the transfected cells were pretreated with EC-18 (0.01-10 µg/ml) at the indicated concentrations and incubated for 24 h, followed by stimulation with 10 ng/ml of IL-4 and IFN-γ for an additional incubation period of 6 h. STAT6 and STAT1 promoter activities were assessed using the Dual-GloTM Luciferase assay system. Each bar represents the mean±SD. Significant values are represented by *(p<0.05) and **(p<0.001). It is a comparison of the EC-18- treated cells to IL-4 alone. |
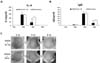 | Figure 6EC-18 decreased the plasma levels of IL-4 and IgE in DNCB-induced atopy-like dermatitis mice. EC-18 was administrated orally to the mice every day for 18 days and then sensitized with 0.2% DNCB 6 times and 1% DNCB 1 time over 7 days and then challenged with 0.2% DNCB for 5 times every 2 days. After the collection of serum from mice at Day 0, Day 4, Day 14, and Day 18, the plasma IL-4 and IgE levels were analyzed. Each bar represents the mean±SD. Significance (p<0.05), indicated by *, is in the EC-18 -treated group versus the control group treated with olive oil. |
ACKNOWLEDGEMENTS
This work was supported by the KRIBB Research Initiative Program (KGM4701511) of the Republic of Korea and a grant (IGM0161411) from ENZYCHEM Lifesciences.
References
1. Kleiman R, Miller RW, Earle FR, Wolff IA. Optically active aceto-triglycerides of oil from Euonymus verrucosus seed. Lipids. 1966; 1:286–287.

2. Myher JJ, Kuksis A, Marai L, Sandra P. Identification of the more complex triacylglycerols in bovine milk fat by gas chromatography-mass spectrometry using polar capillary columns. J Chromatogr. 1988; 452:93–118.

3. Limb JK, Kim YH, Han SY, Jhon GJ. Isolation and characterization of monoacetyldiglycerides from bovine udder. J Lipid Res. 1999; 40:2169–2176.

4. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004; 52:874–878.

5. Lee TS, Yook JS, Lee JS, Yoo CH, Lee JC, Lee CM, Lee WH. Preparation of glycerol derivatives and intermediates therefor. KR 10-0789323. 2005.
6. Lee TS, Yook JS, Lee JS, Yoo CH, Lee JC, Lee CM, Lee WH. Preparation method of 1-palmitoyl-3-acetylglycerol, and preparation method of 1-palmitoyl-2-linoleoyl-3-acetylglycerol using same. WO 2013/043009 A2. 2013.
7. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004; 27:1121–1125.

8. Kim MH, Chang HM, Kim TW, Lee SK, Park JS, Kim YH, Lee TY, Jang SJ, Suh CW, Lee TS, Kim SH, Lee SG. EC-18, a synthetic monoacetyldiacylglyceride, inhibits hematogenous metastasis of KIGB-5 biliary cancer cell in hamster model. J Korean Med Sci. 2009; 24:474–480.

9. Hong JJ, Koh Y, Park JS, Jung HD, Kim SH, Lee TS, Badellino MM. Enteral administration of a synthetic monoacetyldiglyceride improves survival in a murine model of abdominal sepsis. J Trauma. 2010; 68:62–68.

10. Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn KY, Lee TS, Kim JW, Ahn KS, Oh SR. EC-18, a synthetic monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol), attenuates the asthmatic response in an aluminum hydroxide/ovalbumin-induced model of asthma1. Int Immunopharmacol. 2014; 18:116–123.

12. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013; 31:317–343.

13. Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010; 184:3186–3190.

14. Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, Katagiri K, Fujiwara S. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. 2013; 22:30–35.

15. Bao L, Shi VY, Chan LS. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol Immunol. 2012; 50:91–97.

17. Zhu N, Gong Y, Chen XD, Zhang J, Long F, He J, Xia JW, Dong L. Association between the polymorphisms of interleukin-4, the interleukin-4 receptor gene and asthma. Chin Med J (Engl). 2013; 126:2943–2951.
18. Nie W, Zang Y, Chen J, Xiu Q. Association between interleukin-4 receptor alpha chain (IL4RA) I50V and Q551R polymorphisms and asthma risk: an update meta-analysis. PLoS One. 2013; 8:e69120.
19. Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol. 2013; 13:351–361.

20. Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013; 38:570–580.

21. Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol. 2013; 6:740–750.

22. Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996; 4:313–319.

23. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007; 96:41–101.
24. Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009; 31:539–550.

25. Wick KR, Berton MT. IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol Immunol. 2000; 37:641–652.

26. Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013; 145:403–415.

27. Gilbey A, Perezgonzalez JD. Health benefits of deer and elk velvet antler supplements: a systematic review of randomised controlled studies. N Z Med J. 2012; 125:80–86.
28. Mushaben EM, Kramer EL, Brandt EB, Khurana Hershey GK, Le Cras TD. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J Immunol. 2011; 187:5756–5763.

29. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003; 3:721–732.

30. Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004; 4:978–988.

31. Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004; 20:429–440.

33. Yang XO, Zhang H, Kim BS, Niu X, Peng J, Chen Y, Kerketta R, Lee YH, Chang SH, Corry DB, Wang D, Watowich SS, Dong C. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol. 2013; 14:732–740.

34. Knosp CA, Carroll HP, Elliott J, Saunders SP, Nel HJ, Amu S, Pratt JC, Spence S, Doran E, Cooke N, Jackson R, Swift J, Fitzgerald DC, Heaney LG, Fallon PG, Kissenpfennig A, Johnston JA. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J Exp Med. 2011; 208:1523–1531.

35. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010; 11:889–896.

36. Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat Immunol. 2006; 7:539–541.

37. Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012; 129:1506–1514.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download