Abstract
Bone marrow-derived mesenchymal stem cells (MSCs) are multipotent, with the ability to differentiate into different cell types. Additionally, the immunomodulatory activity of MSCs can downregulate inflammatory responses. The use of MSCs to repair injured tissues and treat inflammation, including in neuroimmune diseases, has been extensively explored. Although MSCs have emerged as a promising resource for the treatment of neuroimmune diseases, attempts to define the molecular properties of MSCs have been limited by the heterogeneity of MSC populations. We recently developed a new method, the subfractionation culturing method, to isolate homogeneous human clonal MSCs (hcMSCs). The hcMSCs were able to differentiate into fat, cartilage, bone, neuroglia, and liver cell types. In this study, to better understand the properties of neurally differentiated MSCs, gene expression in highly homogeneous hcMSCs was analyzed. Neural differentiation of hcMSCs was induced for 14 days. Thereafter, RNA and genomic DNA was isolated and subjected to microarray analysis and DNA methylation array analysis, respectively. We correlated the transcriptome of hcMSCs during neural differentiation with the DNA methylation status. Here, we describe and discuss the gene expression profile of neurally differentiated hcMSCs. These findings will expand our understanding of the molecular properties of MSCs and contribute to the development of cell therapy for neuroimmune diseases.
Adult stem cells have lineage-restricted differentiation potential. They are generally named according to their tissue of origin, for example, as mesenchymal stem cells (MSCs), hematopoietic stem cells, and endothelial progenitor cells. MSCs have been isolated from various mesenchymal tissues, including bone marrow (BM), adipose tissue, and umbilical cord blood (1,2). Because of their multifaceted immunomodulatory functions, MSCs have emerged as an attractive therapeutic approach for the treatment of immune diseases (3). A number of studies have shown that MSCs are effective in treating immune-related or inflammatory diseases such as graft-versus-host disease, colitis, and pancreatitis (4-6).
Multilineage plasticity is another property of MSCs. MSCs can differentiate into various cell types found in mesenchymal tissues, such as osteocytes, chondrocytes, and adipocytes (7). MSCs can differentiate into ectodermal, endodermal, and mesodermal lineages (8,9). MSCs have been used to repair spinal cord injury in vivo and to promote neuronal recovery (10). In addition, MSCs can adopt neuronal and glial phenotypes in vitro under the appropriate conditions (11,12). Whether the neuronal morphology observed during the neurogenic differentiation of MSCs is an artifact induced by the neurogenic medium is controversial (13,14), but several studies have presented evidence supporting the neural differentiation potential of MSCs (15,16).
Furthermore, MSCs are considered a potent therapeutic tool likely to have practical use in the near future. There have been extensive efforts over the past decade to treat various incurable diseases using stem cells, including neuroimmune disorders such as spinal cord injury, stroke, and multiple sclerosis (17). Because they are multipotent and immunomodulatory, neural stem cells or neural precursor cells are a therapeutic option for the treatment of neuroimmune diseases (18,19). However, considering the simplicity of stem cell isolation, the ease of cell expansion, and the wide range of applicability, MSCs offer a good alternative for the treatment of neuroimmune diseases, particularly given their neuroglial potential and immunomodulatory properties. Therefore, a detailed characterization of neurally differentiated MSCs is needed to improve cell-based treatments for neuroimmune diseases.
The density-gradient centrifugation method is the most popular technique for isolating MSCs from BM (20). MSCs obtained by this method are heterogeneous because they contain mixed populations of MSCs. Although all of the cells possess MSC characteristics, their cell surface marker expression, differentiation potential, and cytokine secretion vary, suggesting that BM contains a variety of MSC populations with different biological capacities (21). Studies of heterogeneous MSCs have been informative, but the heterogeneity of the cell population likely affects the interpretation of data acquired from experiments with these cells. In light of this shortcoming, we recently developed a novel method, the subfractionation culturing method (SCM), to isolate and establish homogeneous human clonal MSCs (hcMSCs) from small aspirates of human BM (21). hcMSC lines established with our protocol express well-known MSC markers and differentiate into various cell types, including osteocytes, chondrocytes, and adipocytes. Additionally, the hcMSC clones express neural or hepatocytic phenotypes after neural or hepatogenic differentiation in vitro.
Although several microarray analyses have examined the expression profiles of MSCs (22,23), we could not find any studies that analyzed gene expression in homogeneous clonal MSCs during differentiation. Because a number of MSC populations with different differentiation potentials might be present in the BM (21), we used hcMSCs with high neurogliogenic potency to compare gene expression before and after neural differentiation. In this study, we verified the neural potency of highly homogeneous hcMSCs and used microarray analysis to assess gene expression during their differentiation into neuroglial cells. Additionally, we performed CpG methylation array analysis and compared methylation status with gene expression to obtain a more reliable profile of gene expression in neurally differentiated hcMSCs.
BM aspirates were taken from the iliac crest of a healthy male donor after informed consent was provided (approved by the INHA University Medical School Institutional Review Board; IRB Number 10-51). Isolation of hcMSCs was carried out as previously described (21). Several cell surface antigens on the established hcMSC line, named KBHD502, were characterized by flow cytometry. The antibodies used for the analysis were anti-CD14, anti-CD29, anti-CD31, anti-CD34, anti-CD44, anti-CD73, anti-CD90, anti-CD105, anti-CD119, anti-CD133, anti-CD166, anti-HLA class I, anti-HLA-DR, anti-Stro-1, anti-c-Met, and anti-c-Kit (BD Biosciences Pharmingen, San Diego, CA, USA). The cells were analyzed in a FACSCalibur flow cytometer (BD Biosciences). Isotype-matched control antibodies were used as controls.
The in vitro immunosuppressive activity of hcMSCs was determined by [3H]-thymidine incorporation. Briefly, 2×105 peripheral blood mononuclear cells (PBMCs) from two different healthy donors (1×105 cells each) were mixed and cultured in a 96-well plate for mixed lymphocyte reactions. hcMSCs (4×104 cells) were co-cultured at a ratio of 1:5 (hcMSCs: PBMCs) in these reactions. [3H]-thymidine (1µCi/reaction) was added for the last 12~16 h of culture. Radioactivity was measured in a beta-counter.
For IF staining, the cells were seeded onto an 8-well chamber slide (Nunc, Naperville, IL, USA) at a density of 1×104 cells/well. After a 24-h incubation, the growth medium was removed and replaced with neurogenic differentiation medium (neurobasal medium supplemented with B27 supplement (Gibco-BRL, Gaithersburg, MD, USA), 1 mM dibutyryl cAMP (Sigma-Aldrich, St. Louis, MO, USA), 0.5 mM 1-methyl-3-isobutylxanthine (Sigma-Aldrich), 20 ng/ml human epidermal growth factor (Sigma-Aldrich), 40 ng/ml basic fibroblast growth factor (Sigma-Aldrich), 10 ng/ml fibroblast growth factor 8 (Peprotech, Rocky Hill, NJ, USA), and 10 ng/ml brain-derived neurotrophic factor (R&D Systems, Minneapolis, MN, USA). The cells were cultured in serum-free neurogenic differentiation medium for 2 weeks. At the end of the differentiation period, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% TritonX-100/PBS. The cells were labeled with primary antibodies (1:200~:1,000), including rabbit anti-human glial fibrillary acidic protein (GFAP; Sigma-Aldrich), mouse anti-neuron-specific class III β-tubulin (Tuj1; Millipore, Billerica, MA, USA), and rabbit anti-microtubule- associated protein 2 (MAP2; Millipore) overnight at 4℃. After incubation with the primary antibodies, the cells were incubated for 1 h with AlexaFluor488- or AlexaFluor594-conjugated secondary antibodies (1:300; Molecular Probes, Carlsbad, CA, USA). The cells were subsequently stained with 4',6-diamidino-2-phenylindole (DAPI; Molecular Probes) or propidium iodide (PI; Molecular Probes) for 1 min. After mounting, the samples were analyzed by confocal microscopy (Zeiss LSM510 Meta Confocal Imaging System; Carl Zeiss, Thornwood, NY, USA). In vitro adipogenic, chondrogenic, hepatogenic, and osteogenic differentiation are described in the supplemental material (Supplemental Method 1).
At the end of neurogenic differentiation, total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using the Reverse Transcription System (Promega, Madison, WI, USA). Semi-quantitative RT-PCR was performed using ExTaq DNA polymerase (Takara Biotechnology, Shiga, Japan). Amplified PCR products were electrophoresed on 1% agarose gels. PCR primer sequences are summarized in the supplemental material (Supplemental Method 2).
At the end of the neurogenic differentiation period, total RNA was isolated using TRIzol reagent. The synthesis of Target cRNA probes were synthesized and hybridized using Agilent's Low RNA Input Linear Amplification kit (Agilent Technology, Palo Alto, CA, USA) according to the manufacturer's instruction. The Cy3/5-labeled cRNA amplicon was purified on a cRNA Cleanup Module (Agilent Technology). Labeled cRNA was quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). After checking the labeling efficiency, the cRNA was fragmented by adding blocking agent and fragmentation buffer and incubating at 60℃ for 30 min. The cRNA fragments were resuspended in hybridization buffer and directly pipetted onto Agilent's Whole Human Genome Oligo Microarray (44K). The arrays were hybridized at 65℃ for 17 h in an Agilent Hybridization oven (Agilent Technology). According to the manufacturer's protocol, the hybridized microarrays were washed. For microarray data acquisition and analysis, the hybridized arrays were scanned with Agilent's DNA microarray scanner and quantified with the Feature Extraction software (Agilent Technology). Data was normalized, and regulated genes were identified with GeneSpring GX 7.3.1 (Agilent Technology). The averages of the normalized ratios were calculated by dividing the average of the normalized signal channel intensity by the average of the normalized control channel intensity. Functional annotation of genes was carried out according to the guidelines of the Gene Ontology™ Consortium (http://www.geneontology.org/index.shtml) using GeneSpring GX 7.3.1.
For CpG methylation array analysis, genomic DNA samples from control and neurogenically differentiated hcMSCs were extracted using a Wizard Genomic DNA Purification kit (Promega). Genomic DNA was sonicated to an average fragment length of 300 bp using a VCX130 ultrasonic processor (Sonics & Materials, Newtown, CT, USA) and purified using a QIAquick PCR Purification kit (Qiagen, Valencia, CA, USA). To isolate methylated genomic DNA, 2µg of His6-tagged methyl DNA-binding protein was incubated with 500 ng of sheared genomic DNA fragments from control and differentiated hcMSCs for 8 h at 4℃. The enriched methylated DNA was amplified using a Whole Genome Amplification kit (Sigma-Aldrich). The amplified products from control and differentiated cells were labeled with Cy3-dUTP and Cy5-dUTP, respectively, using the BioPrime Total for Agilent aCGH system (Invitrogen). The labeled DNA samples were mixed and hybridized to a human 244K CpG island microarray (Agilent Technology). The slides were scanned with an Agilent scanner, and the images were quantified with the Feature Extraction software (Agilent Technology). Two independent experiments were performed. Data were normalized and differentially regulated genes were identified using GeneSpring GX 7.3.1 software (Agilent Technology).
We isolated and established several hcMSCs from a healthy donor's BM by SCM. One clone exhibited excellent multilineage plasticity. The clonal cells expressed known MSC markers, including CD29, CD44, CD73, CD90, CD105, CD133, CD166, HLA-class I, and Stro-1; however, they were negative for hematopoietic and endothelial markers, including CD14, CD31, CD34, and HLA-DR (Fig. 1A). Additionally, the cells were positive for c-MET, but negative for CD119 and c-Kit. The hcMSCs significantly inhibited T cell proliferation in mixed lymphocyte reactions, suggesting that they have intrinsic immunomodulatory activity (Fig. 1E). As expected, the cells successfully transdifferentiated into adipocytes, chondrocytes, hepatocytes, and osteocytes when induced in vitro with adipogenic, chondrogenic, hepatogenic, and osteogenic medium, respectively (Fig. 1B). Additionally, the cells exhibited a neural phenotype when cultured in the appropriate induction medium. After 14 days of differentiation, the cells were fixed and stained for IF analysis. When examined by confocal microscopy, the induced cells were positive for the neuronal and glial markers Tuj1, MAP2, and GFAP (Fig. 1C). GFAP, neurofilament M (NFM), nestin, MAP2, and Hes1 mRNA levels increased during neural differentiation (Fig. 1D). The mRNA levels of these neuroglial maker genes were increased or maintained for up to 21 days. These results showed that the hcMSC line could acquire a neural phenotype after appropriate induction.
Although neuronal expression profiles of BM-derived MSCs have been reported (22,24), to our knowledge, no study has assessed a homogeneous MSC population. We chose homogeneous hcMSCs rather than heterogeneous MSC pools in order to improve the reliability of the gene expression data and to eliminate the mixed information obtained from a heterogeneous MSC population. Two independent RNA samples from the hcMSC line were isolated before and after neural induction for 14 days and then subjected to microarray analysis. The average value of the two independent results was used for data analysis.
The microarray probe set included 27,958 genes. A 2-fold increase over expression in control cells was selected as the threshold for upregulated genes, and a 2-fold decrease was selected as the threshold for downregulated genes. With these criteria, we identified 1025 upregulated genes and 1415 downregulated genes. Table I shows the top 30 upregulated genes and the top 30 downregulated genes in differentiated MSCs. During neural differentiation, the expression of PCSK1, MMP10, and CXCL3 increased 42.3-fold, 30.1-fold, and 24.4-fold, respectively. Conversely, ITGA8, DUSP18, and DKK1 decreased 23.2-fold, 19.4-fold, and 17.8-fold, respectively. The genes were sorted and classified into several groups according to gene ontology. We examined the data with respect to neuronal differentiation or nervous system differentiation (Supplemental Table). Forty-three genes, including Ereg, neurotrophic tyrosine kinase receptor 3 (NTrk3), Musashi homolog 1 (MSI1), Sema4G, NDRG2, Amigo1, EphrinB1, Notch3, and Pax6 were upregulated, whereas only 3 genes (MyD88, GDAP2, and RQCD1) were downregulated. NTrk3, Notch3, NDRG2, MSI1, Amigo1, Sema4G, and EphrinB1 play important roles in neurogenesis. The fact that these upregulated genes are involved in neuronal differentiation and nervous system development provides evidence of the genuine neurogenic potential of MSCs, refuting some controversial studies that suggest a false-positive gain of neural phenotype in MSCs treated with neural induction reagents.
To verify the gene expression profile generated by the microarray analysis, genes were randomly selected for evaluation by RT-PCR (Fig. 2). The expression of PCSK, Ereg, MMP10, Cdx1, Wnt7a, Sox4, and MEPE increased, whereas the expression of Smad3, Smurf2, NEDD4L, STAT1, DAAM2, ITGA6, and DKK1 decreased. These results were consistent with the microarray data, indicating that our microarray experiments were highly reliable.
To generate additional gene expression data, we examined epigenetic changes by analyzing promoter methylation and compared the results with the gene expression profile. DNA methylation is a key epigenetic regulatory mechanism for development and gene expression (25,26) that typically occurs at CpG dinucleotides. Clusters of CpG dinucleotides, called CpG islands, are usually found in mammalian gene promoters (27). Methylation of the CpG islands in a promoter region inhibits gene expression, whereas lack of methylation permits gene expression (28). Therefore, examining the CpG methylation status of promoters may provide further insight into gene expression during the neural differentiation of MSCs. We isolated genomic DNA from hcMSCs before and after neural induction for 14 days and used CpG methylation microarray analysis to determine the genome-wide methylation profile.
In differentiated hcMSCs, 1141 promoters were hypermethylated (≥2-fold increase) and 1276 promoters were hypomethylated (≥2-fold decrease) when compared with promoters in control hcMSCs. Among the hypomethylated genes, we looked for genes that were upregulated ≥2-fold in the microarray data. Similarly, among the hypermethylated genes, we looked for genes that were downregulated ≥2-fold in the microarray data. We identified 50 genes whose expression was upregulated and whose promoters were hypomethylated, along with 68 genes with downregulated expression and hypermethylated promoters (Table II). The genes that were upregulated and hypomethylated included NTrk3, Sox4, SYTIII, FABP5, GRIA4, NXPH4, FoxC1, BARHL2, PAPLN, and Pax6. Among these genes, NTrk3 and Sox4 belonged to the group of 30 genes with the highest expression in differentiated hcMSCs, as listed in Table I. Most of genes in Table II are involved in neurogenesis or nervous system development, confirming the high fidelity of our microarray data. Genes that were downregulated and hypermethylated included NRXN3, Cdc42EP3, NEDD4L, SIRPα, and DEK.
Microarray technology enables easy comparison of the differential expression of a large number of genes. However, false-positive results present an obstacle to microarray data analysis. Given that microarray experiments are likely to show variation in their results, sample quality is another critical factor in the analysis. Some studies have determined the gene expression profiles of neural differentiation in MSCs using microarray analysis (16,24). The most common method for isolating MSCs is Ficoll-mediated density gradient centrifugation. The resulting MSC pool is heterogeneous because no additional purification process is used in this method. Even the defined sorting method, which uses specific antibodies, might not completely eliminate heterogeneity in the isolated MSC population. We previously developed an easy and simple isolation method for obtaining hcMSCs called SCM (21). Because no reports have described microarray analysis of a homogeneous MSC population, we examined the gene expression profile of neurally differentiated hcMSCs. The microarray results were confirmed with RT-PCR analysis. In addition, we combined the microarray data with independent promoter methylation chip array data to generate more accurate information.
According to the gene ontology analysis, the expression of neuronal-specific genes involved in neuronal differentiation and nervous system differentiation increased in neurally differentiated hcMSCs (Supplemental Table). Genes involved in synaptic transmission, such as EGR3 (listed in Supplemental Table), SYT3 (listed in Table II), nicotinic cholinergic receptor 5 (not listed, 4.5-fold increase), glycine receptor alpha 3 (not listed, 4.4-fold increase), protocadherin beta 13 (not listed, 3.1-fold increase), neurotensin receptor 1 (not listed, 2.1-fold increase), and a voltage-dependent calcium channel (not listed, 2.1-fold increase), were upregulated. Genes encoding channel proteins, including a voltage-gated potassium channel, a glycine receptor, glutamate receptors, nicotinic cholinergic receptors, and a voltage-dependent calcium channel, were also upregulated (not listed).
The canonical Wnt signaling pathway plays an important role in neurogenesis (29,30). According to our microarray analysis, the Wnt signaling molecule Wnt7a was highly upregulated at the mRNA level, whereas DKK1, a Wnt signaling inhibitor, was significantly downregulated 17.8-fold (Table I). This is consistent with reports describing the functional role of Wnt signaling in neurogenesis (31,32). With regard to Wnt signaling molecules, the genes encoding Axin2 and FZD10 were upregulated, and those encoding TLE4 and DACT1 were downregulated in our microarray analysis (not listed). These results are consistent with those of a recent study demonstrating that FZD10 promotes the development of sensory neurons (33).
One of the interesting gene groups identified in this study comprised E3 ubiquitin protein ligases, which catalyze protein ubiquitination (34,35). Protein ubiquitination is catalyzed by three groups of enzymes, ubiquitin-activating E1 enzymes, ubiquitin-conjugating E2 enzymes, and ubiquitin-ligating E3 ligases. Functionally, the E3 ligase determines which proteins are ubiquitinated. There are two types of E3 ligases: the RING type and the HECT type (36,37). In humans, most E3 ligases are RING-type enzymes; only 28 ligases are HECT-type enzymes, such as those in the HERC family and NEDD4 family (36,37). In neurally differentiated hcMSCs, the gene encoding NEDD4L was downregulated and its promoter was hypermethylated (Table II). Other E3 ubiquitin protein ligase genes, including Smurf2 (not listed, 4.2-fold decrease), WWP2 (not listed, 2.3-fold decrease), and NEDD4 (not listed, 6.4-fold decrease), were also downregulated when assessed by microarray analysis. Notably, these genes belong to the NEDD4 family of HECT-type E3 ligases (37). Ubiquitination of Rap1B by Smurf2 is required for neuronal polarity (38). Downregulation of Smurf2 expression was confirmed by RT-PCR analysis (Fig. 2). Smurf1 regulates neurite extension through Rho GTPase (39). On the other hand, the expression of other E3 ligase genes increased, including TRIM50 and RNF25 (data not shown). Interestingly, TRIM50 and RNF25 are members of the RING-type E3 ligase family. Future studies will determine whether downregulation of specific HECT-type E3 ubiquitin ligases, such as NEDD4L and Smurf2, is functionally correlated with the acquired neuronal phenotype of MSCs. Smurf2 might be a good candidate for future study because its downregulated expression was verified by RT-PCR (Fig. 2).
Finally, it is notable that TNFAIP6 (16.8-fold increase) and IL1RN (16.5-fold increase) were highly upregulated in neurally differentiated hcMSCs (Table I). TNFAIP6, also called TSG-6, plays a critical role in MSC-mediated immunosuppression (40). TNFAIP6 reduces various inflammatory conditions, including myocardial infarction, zymosan-induced peritonitis, and corneal injury (41-43). IL1RN encodes interleukin-1 receptor antagonist (IL-1Ra), which can modulate various IL-1-related inflammatory responses by inhibiting IL-1α and IL-1β (44). Anakinra, an approved IL-1Ra drug used to treat rheumatoid arthritis, has therapeutic effects on a variety of local, systemic, hereditary autoimmune inflammatory diseases (45).
We evaluated the gene expression profile of neurally differentiated MSCs using homogeneous clonal cells. To verify the gene expression profile, we confirmed the results from the microarray analysis with RT-PCR and analyzed data obtained from CpG methylation microarray analysis. Our results provide evidence for the neuronal and glial potency of MSCs. Thus, MSCs can be used for the development of cell therapies that target neuroimmune disorders such as stroke, multiple sclerosis, and Parkinson's disease. The upregulation of some anti-inflammatory genes, such as TNFAIP6 and IL1RN, during neural differentiation improves the outlook on the therapeutic utility of MSCs. Furthermore, neurogenically induced or neurogenically primed hcMSCs retained their immunosuppressive activity against activated T cells in vitro (unpublished data). Thus, an optimized balance between the immunomodulatory activity and neurogenic potency of MSCs may provide a new therapeutic solution for incurable neuroimmune diseases. Although our study is only one small step toward understanding the biological and molecular properties of MSCs, further investigation should contribute to the development of stem cell therapy.
Figures and Tables
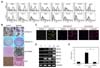 | Figure 1Characterization of hcMSCs and neural differentiation. The hcMSC line was established by SCM. (A) The cell surface markers were analyzed by flow cytometry. The cells were positive for CD29, CD44, CD73, CD90, CD105, CD133, CD166, HLA class I, Stro-1, and c-Met; however, they were negative for CD14, CD31, CD34, CD119, HLA-DR, and c-Kit. Thus, hcMSCs expressed the cell surface antigens typical of MSCs. (B) To determine the multilineage differentiation potential of the hcMSCs, the cells were cultured in adipogenic, chondrogenic, hepatogenic, and osteogenic medium to induce differentiation. Cytochemical staining showed that the cells differentiated into adipocytes (evaluated with red lipid droplets by oil red O), chondrocytes (evaluated with red glycosaminoglycans by safranin O), hepatocytes (evaluated with red glycogen deposits by periodic acid-Schiff (PAS)), and osteocytes (evaluated with black calcified nodules by von Kossa), respectively. (C) To examine the neural differentiation potential of hcMSCs, the cells were induced for 14 days, and neural marker expression was analyzed by IF staining. The differentiated cells exhibited neuronal and glial morphology and expressed several neural markers (GFAP, Tuj1, and MAP2), indicating that the hcMSC line has neural differentiation potential, as well as adipogenic, chondrogenic, hepatogenic, and osteogenic differentiation potential. (D) To confirm the neural differentiation of the hcMSCs at the molecular level, mRNA expression was analyzed. RT-PCR showed that the expression of neuronal and glial marker genes, including NF-M, nestin, MAP2, GFAP, and Hes1, increased during neural differentiation. (E) To assess the immunosuppressive activity of hcMSCs, lymphocyte proliferation in a mixed lymphocyte reaction was measured by [3H]-thymidine incorporation in the presence of hcMSCs. hcMSCs significantly inhibited the proliferation of activated lymphocytes when co-cultured at a ratio of 1:5 (hcMSCs:PBMCs). (-) ctrl, negative control; *p=0.01. |
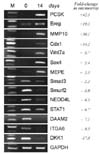 | Figure 2RT-PCR confirmation of the gene expression changes observed in the microarray analysis of neurally differentiated hcMSCs. After the hcMSCs were differentiated for 14 days, mRNA was extracted and subjected to microarray analysis. To confirm the microarray data, the expression of randomly selected genes that changed 2-fold or more in the microarray analysis was assessed by RT-PCR. + denotes increase and - represents decrease. |
ACKNOWLEDGEMENTS
This study was supported by the Bio & Medical Technology Development Program (NRF-2011-0019634 and NRF-2011-0019637) of the National Research Foundation of the Korean government (MEST), and by a grant from Inha University (44773-01).
References
1. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow; Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–247.
2. Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009; 1176:101–117.

3. Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012; 35:213–221.

4. Yoo HS, Yi T, Cho YK, Kim WC, Song SU, Jeon MS. Mesenchymal stem cell lines isolated by different isolation methods show variations in the regulation of graft-versus-host disease. Immune Netw. 2013; 13:133–140.

5. Anderson P, Souza-Moreira L, Morell M, Caro M, O'Valle F, Gonzalez-Rey E, Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013; 62:1131–1141.

6. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.

7. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.

8. Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005; 102:186–191.

9. Tao XR, Li WL, Su J, Jin CX, Wang XM, Li JX, Hu JK, Xiang ZH, Lau JT, Hu YP. Clonal mesenchymal stem cells derived from human bone marrow can differentiate into hepatocyte-like cells in injured livers of SCID mice. J Cell Biochem. 2009; 108:693–704.

10. Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002; 99:2199–2204.

11. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370.

12. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001; 282:148–152.

13. Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004; 77:192–204.

14. Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004; 77:174–191.

15. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, Medico E, Madon E, Vercelli A, Fagioli F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006; 34:1563–1572.

16. Kim S, Honmou O, Kato K, Nonaka T, Houkin K, Hamada H, Kocsis JD. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006; 1123:27–33.

17. Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010; 16:203–209.

18. Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007; 61:209–218.

20. Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996; 11:312–324.

21. Song SU, Kim CS, Yoon SP, Kim SK, Lee MH, Kang JS, Choi GS, Moon SH, Choi MS, Cho YK, Son BK. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008; 17:451–461.

22. Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008; 9:166.

23. Shakhbazau AV, Goncharova NV, Kosmacheva SM, Kartel NA, Potanev MP. Plasticity of human msesnchymal stem cell phenotype and expression profile under neurogenic conditions. Bull Exp Biol Med. 2009; 147:513–516.

24. Yamaguchi S, Kuroda S, Kobayashi H, Shichinohe H, Yano S, Hida K, Shinpo K, Kikuchi S, Iwasaki Y. The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC)-a preliminary study using microarray analysis. Brain Res. 2006; 1087:15–27.

25. Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001; 293:1089–1093.

26. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003; 33:Suppl. 245–254.

27. Larsen F, Gundersen G, Lopez R, Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992; 13:1095–1107.

28. Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999; 99:451–454.

29. Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006; 133:4451–4461.

30. Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008; 11:1383–1391.

31. Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008; 86:281–296.

32. Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retroelements during adult neurogenesis. Nat Neurosci. 2009; 12:1097–1105.

33. Garcia-Morales C, Liu CH, Abu-Elmagd M, Hajihosseini MK, Wheeler GN. Frizzled-10 promotes sensory neuron development in Xenopus embryos. Dev Biol. 2009; 335:143–155.

36. Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling. PLoS One. 2008; 3:e1487.

37. Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009; 10:398–409.

38. Schwamborn JC, Muller M, Becker AH, Puschel AW. Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 2007; 26:1410–1422.

39. Bryan B, Cai Y, Wrighton K, Wu G, Feng XH, Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005; 579:1015–1019.

40. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012; 20:14–20.

41. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009; 5:54–63.

42. Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH Jr, Prockop DJ. Antiinflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010; 107:16875–16880.

43. Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011; 118:330–338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download