Abstract
K/BxN serum can transfer arthritis to normal mice owing to the abundant autoantibodies it contains, which trigger innate inflammatory cascades in joints. Little is known about whether gut-residing microbes affect host susceptibility to autoantibody-mediated arthritis. To address this, we fed C57BL/6 mice with water containing a mixture of antibiotics (ampicillin, vancomycin, neomycin, and metronidazol) for 2 weeks and then injected them with K/BxN serum. Antibiotic treatment significantly reduced the amount of bacterial genomic DNA isolated from fecal samples, in particular a gene encoding 16S ribosomal RNA derived from segmented filamentous bacteria. Arthritic signs, as indicated by the arthritic index and ankle thickness, were significantly attenuated in antibiotic-treated mice compared with untreated controls. Peyer's patches and mesenteric lymph nodes from antibiotic-treated mice contained fewer IL-17-expressing cells than those from untreated mice. Antibiotic treatment reduced serum C3 deposition in vitro via the alternative complement pathway. IL-17-/- congenic C57BL/6 mice were less susceptible to K/BxN serum-transferred arthritis than their wild-type littermates, but were still responsive to treatment with antibiotics. These results suggest that gut-residing microbes, including segmented filamentous bacteria, induce IL-17 production in GALT and complement activation via the alternative complement pathway, which cause the host to be more susceptible to autoantibody-mediated arthritis.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that mainly affects the synovial membranes, cartilage, and bone of diarthrodial joints (1). Patients with RA are characterized by an abundance of autoantibodies, such as rheumatoid factors and anti-cyclic citrullinated peptide antibodies. Production of autoantibodies requires activation of self-reactive T and B cells, which probably stems from breakdown of self-tolerance. Such autoantibodies play a central role in joint destruction by triggering the inflammatory responses mediated by Fc receptors (FcR) and complement factors. These pathogenic processes are relatively well reproduced in the K/BxN mouse model of RA.
K/BxN TCR transgenic mice spontaneously develop severe arthritis, which requires the transgenic T cells that specifically recognize a self-peptide derived from glucose-6-phosphate isomerase (GPI) in the context of MHC I-Ag7 and their cognate B cells (2). Plasma cells secreting GPI-specific Abs are crucial for triggering the disease, as evidenced by the fact that K/BxN serum alone can transfer the disease to naive mice (3). Autoantibodies abundant in K/BxN serum trigger innate inflammatory cascades in the joints, and adaptive immune responses are for this transient process (4). Therefore, in this model, adaptive autoimmune responses represent the initiation phase of pathogenesis, which is followed by the distal effector phase, mediated by autoantibody-mediated innate inflammatory responses. Using the model of K/BxN serum transferred arthritis, we and other investigators have identified many genetic elements that are important for the innate effector function stage of arthritis (5,6). This stage requires the activities of IL-1-β and TNF-α, but not of IL-6 or IL-21. However, the influence of environmental factors on this stage largely remains to be defined.
The gastrointestinal microbiota community is one of the most significant influences on the mucosal immune system in both humans and mice; the mucosal immune system seems to crosstalk continuously with gastrointestinal microbes (7). This interaction can alter the immune system either beneficially or detrimentally for the host. For example, gut microbes contribute to the normal shaping of GALT, as evidenced by defective lymphocyte compartments and reduced levels of antibodies in the GALT of germ-free mice (8-10). By contrast, gut-residing microbes can also perturb the steady state of GALT, causing the host to be susceptible to inflammatory diseases (11,12). For example, introducing segmented filamentous bacteria (SFB) into the gut of germ-free mice hastened the onset of arthritis by more than 5 weeks in K/BxN mice (13). This phenomenon was mediated by the induction of Th17 cells in the lamina propria. Although this study demonstrated that SFB activates the initiation phase of autoimmune arthritis, little is known about whether gut microbes also influence the effector phase of autoimmune arthritis.
To address this issue, we undertook the present study using the model of K/BxN serum-transferred arthritis. We treated wild-type (WT) and IL-17-/- mice with antibiotics to deplete all Gram-positive, Gram-negative, and anaerobic bacteria from their guts. We found that mice treated with antibiotics were significantly less susceptible to serum-transferred arthritis than untreated mice, and that this susceptibility is probably conferred by both IL-17-dependent and -independent mechanisms. Our results suggest that gut-residing bacteria, including SFB, confer increased susceptibility to the host to antibody-mediated inflammatory responses in the site where antibodies deposit.
K/BxN mice were generated by crossing KRN TCR-transgenic mice on a C57BL/6 background with NOD mice (2). IL-17-/- C57BL/6 breeders were provided by Dr Y. Iwakura (University of Tokyo, Tokyo, Japan), and their progeny were maintained in the animal facility of Hanyang University. Six-to-seven-week-old male C57BL/6 mice were purchased from Orient-Bio (Seongnam-si, Korea). All mice were kept under specific pathogen-free (SPF) barrier conditions of Hanyang University. The study was approved by institutional Animal Care and Use Committee.
Mice were fed with water containing a mixture of antibiotics for 24 days. The mixture of antibiotics included 0.5 mg/ml ampicillin (Calbiochem; La Jolla, CA, USA), 1 mg/ml neomycin (Calbiochem; La Jolla, CA, USA), 0.2 mg/ml vancomycin (Korea United Pharm; Seoul, Korea), and 1 mg/ml metronidazole (MP Biomedicals; Solon, OH, USA). The antibiotic mixture was replaced on a weekly basis. Serum was collected from 8~12-week-old arthritic K/BxN mice. WT and IL-17-/- C57BL/6 mice that had been treated with antibiotics or vehicle for 14 days were injected intraperitoneally (i.p.) with 200 µl of K/BxN serum. Arthritic symptoms were evaluated every 2 days for a total of 10 days in a blinded manner, and disease severity was evaluated using a previously described scoring system (14). In this system, the maximum score per mouse is 16, and scores are expressed as the mean arthritic index on a given day. The thickness of both hind paw ankles was measured axially across the malleoli using a caliper (Mitutoyo; Kanagawa, Japan).
Fecal pellets were collected from each mouse that had been treated with antibiotics or their vehicles for 14~24 days, and bacterial genomic DNA was purified from the pellets using the Stool DNA Extraction Kit (Bioneer; Seoul, Korea) according to the manufacturer's instructions. The 16S rRNA gene specific for SFB was amplified by PCR and normalized to the level of the total bacterial (EUB) 16S rRNA gene. PCR conditions were as follows: 30 cycles of 94℃ for 30 s, 58℃ for 30 s, and 72℃ for 30 s. The PCR primers had the following sequences: EUB forward, 5'-ACT CCT ACG GGA GGC AGC AGT-3' and EUB reverse, 5'-ATT ACC GCG GCT GCT GGC-3'; SFB forward, 5'-GAC GCT GAG GCA TGA GAG CAT-3' and SFB reverse, 5'-GAC GGC ACG GAT TGT TAT TCA-3'.
Ten days after serum injection, mesenteric lymph nodes (mLNs) and Peyer's patches were harvested from the mice post mortem. To obtain single cell suspensions of lymphocytes, mLNs were treated as described previously (6), and Peyer's patches were ground, digested with Brenzyme Liberase (Roche; Germany) for 45 min at 37℃, and filtered through a 70-µm-pore-sized strainer. Aliquots of the single cell suspensions were stimulated with 20 ng/ml phorbol myristate acetate (PMA) and 1 µM ionomycin (both from Sigma-Aldrich; St. Louis, MO, USA) for 6 h and were surfaceor intracellularly stained as previously described (6), followed by FACS analyses. The mAbs used for this study were as follows: anti-CD4-PerCP and anti-IL-17-PE were purchased from BD Biosciences (San Jose, CA, USA), and anti-IFN-γ-FITC and anti-CD44-APC were purchased from eBioscience (San Diego, CA, USA). Data were acquired with a BD FACS Canto II (BD Biosciences) and analyzed with FACS Diva software.
An aliquot of mouse serum (2 µl) was added to 90 µl PBS containing 107 zymosan particles (ICN Biomedicals; Aurora, OH, USA), 10 mM EGTA, and 5 mM MgCl2, and incubated for 20 min at 37℃. The reaction was stopped with 10 mM EDTA. The zymosan particles were washed with FACS buffer (PBS containing 1% BSA and 0.1% sodium azide), incubated with anti-mouse C3-FITC (ICN Biomedicals), and analyzed by FACS.
Even mice housed under SPF barrier conditions harbor trillions of commensal microbes in their guts. To remove these gut microbes, we treated mice with a mixture of antibiotics including ampicillin, vancomycin, neomycin, and metronidazole. Ampicillin and vancomycin target Gram-positive bacteria; neomycin and metronidazole target Gram-negative and anaerobic bacteria, respectively (13). After feeding the mice with antibiotics for 14 days, fecal pellets were collected from each mouse and assayed to quantify the bacterial genomic DNA. The total amount of bacterial genomic DNA was significantly lower after antibiotic treatment in both WT and IL-17-/- mice, indicating that antibiotics efficiently reduced gut microbiomes in both strains of mice (Fig. 1A). Interestingly, without antibiotic treatment, bacterial genomic DNA levels were significantly lower in the fecal samples from IL-17-/- mice than from WT mice. Therefore, IL-17 deficiency seems to reduce the growth of bacterial colonies in the gut.
Because SFB is known to induce Th17 cells in the lamina propria (13,15), we assessed whether the mice in this study had SFB in their guts by amplifying the 16S rRNA gene specific for SFB. We detected the 16S rRNA gene of SFB in the microbiome isolated from naive WT mouse feces, but not in the microbiome of antibiotic-treated WT mice (Fig. 1B). We also failed to detect the 16S rRNA gene of SFB in fecal samples from IL-17-/- mice, regardless of whether they had been treated with antibiotics. This result demonstrated that antibiotic treatment reduced the residence of gut bacteria and depleted SFB from WT mice. In addition, this finding showed that IL-17 deficiency is associated with the absence of SFB in the gut.
To determine whether gut-residing bacteria affect the ability of autoantibodies to induce innate synovitis, we transferred K/BxN serum to mice pretreated with antibiotics (Fig. 2A). WT mice pretreated with antibiotics had a significantly lower mean arthritic index and mean ankle thickness than untreated WT mice (Fig. 2B). IL-17-/- mice were significantly less susceptible to arthritis induction than WT mice, and arthritic signs were further decreased in response to antibiotics. Therefore, it is likely that depletion of gut-residing microbes causes the host to be less sensitive to innate effector functions of autoantibodies and that these responses are in part mediated by IL-17.
We next assessed whether gut microbes induce the development of IL-17-producing cells in GALT. To achieve this, we observed IL-17-expressing cells within Peyer's patches and mLNs of antibiotic-treated or untreated mice. The proportion of cells extracted from Peyer's patches that produced IL-17 was significantly lower in antibiotic-treated mice than in their untreated counterparts (Fig. 3). By contrast, the fraction of IFN-γ-producing cells was higher after antibiotic treatment, presumably reflecting the antagonism between Th1 and Th17 cells. This bias was also observed in mLNs, albeit to a lesser extent than in Peyer's patches; the proportions of IL-17- and IFN-γ-producing cells in the CD4+CD44+ cell population decreased and increased, respectively, after antibiotics treatment. We did not observe any difference in the proportions of these cytokine-producing cells in joint-draining LNs or in spleens between antibiotic-treated and untreated groups (data not shown). Therefore, gut-residing microbes seem to promote the emergence of IL-17-producing cells specifically in GALT. Because IL-17-/- mice were less arthritic than WT mice, as shown in Fig. 2, IL-17 provision associated with gut microbes might exacerbate antibody-induced joint inflammation.
One of the mechanisms by which autoantibodies cause innate inflammation is complement activation. In particular, the alternative complement pathway, but not the classical complement pathway, is involved in the pathogenesis of K/BxN serum-transferred arthritis (4). We examined whether the reduced susceptibility to arthritis caused by antibiotics was associated with the alternative complement pathway. To this end, serum from antibiotic-treated or untreated mice was assayed to determine the extent of C3 deposition on the surface of zymosan. In WT mice, K/BxN serum transfer increased the fraction of C3-bound zymosan, which was reduced by antibiotic treatment (Fig. 4). However, IL-17-/- mice did not respond to the K/BxN serum transfer in terms of C3 activation. Thus, antibiotic treatment of WT mice reduces the activation of the complement system via the alternative complement pathway that is crucial for induction of serum-transferred arthritis. In addition, this finding demonstrates that IL-17 deficiency is associated with impaired C3 activation.
In the present study, we found that antibiotic-treated mice are more resistant to K/BxN serum-transferred arthritis, suggesting that gut-residing microbes increase the host's susceptibility to autoantibody-mediated innate inflammation. The mechanisms underlying this phenomenon seem to include the induction of IL-17-producing cells, including Th17 cells, in GALT and C3 activation via the alternative complement pathway. We also found that, in addition to antibiotics, IL-17-deficiency causes mice to be less susceptible to the induction of arthritis. Moreover, antibiotic treatment further reduced the arthritic symptoms of IL-17-/- mice. These findings imply that serum-transferred arthritis is mediated by both IL-17-dependent and -independent mechanisms, both of which can be blocked, in part, by the action of antibiotics.
IL-17 can be produced by diverse cell types, including Th17 cells, γδ T cells, mast cells, and innate lymphoid cells (16-18). We identified that the IL-17-producing cells within mLNs were marked as CD4+CD44+, indicative of Th17 cells. However, the cellular source of IL-17 in Peyer's patches was unclear, because this tissue did not contain sufficient CD4+ T cells for analysis. IL-17, regardless of its cellular source, can recruit and activate neutrophils and/or directly provide help to B cells (19-21). Given that neutrophils are known to have an important role in the pathogenesis of serum-transferred arthritis (22), it is likely that gut microbe-driven IL-17 production enables neutrophils to participate in joint inflammation.
The complement system constitutes another mechanism of triggering local tissue inflammation. Microbial infection readily and spontaneously activates and deposits C3 on the surface of microbes, but it was not known whether commensal microbes also activate the complement pathways. We addressed this issue by demonstrating that C3 deposition, which occurred spontaneously in normal naive mice, was decreased in response to antibiotic treatment. Therefore, complements seem to serve as messengers that transfer signals from gut-residing bacteria to the host, leading to the perturbation of immune homeostasis of the host.
There is evidence linking complements with Th17 cells (23-25). Complement C5a promoted autoimmune arthritis in SKG mice by indirectly inducing Th17 cell development (24). However, conflicting results have been reported: C5a reduced Th17 cell differentiation and allergic asthma, while C3a promoted Th17 differentiation (25). Therefore, it appears that the extent of complement activation can have a positive or negative influence on the emergence of Th17 cells depending on the context. We found that complement activity is, in turn, dependent on the presence of IL-17, as shown by the observation that IL-17-deficient mice demonstrated lower levels of C3 deposition than IL-17-replete mice in response to K/BxN serum transfer. Therefore, communication between the complement system and IL-17 might be bidirectional. Bidirectional mutualism was also observed between IL-17 and the gut microbiome. In addition to gut microbiome-driven induction, IL-17 appears to induce bacterial colony expansion, since IL-17-/- mice contained lower amounts of gut microbiome than their WT littermates. This suggests that IL-17 provides a survival niche for bacterial colonization.
In summary, we demonstrate here that antibiotics targeting gut-residing microbes, including SFB, decrease host susceptibility to autoantibody-mediated arthritis via IL-17-dependent and -independent mechanisms. This finding demonstrates that gut-residing microbes regulate the susceptibility of the host to distal effector functions of autoantibody-mediated inflammation in joints.
Figures and Tables
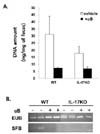 | Figure 1Treatment with antibiotics decreases gut-residing bacteria, including SFB. WT and IL-17-/- (IL-17KO) mice were fed with antibiotics (αB)- or vehicle-containing water for 14~24 days and fecal pellets were collected from each mouse. Bacterial genomic DNA was purified from the fecal samples. (A) The amounts of total bacterial genomic DNA per mg of fecal materials are shown. (B) The 16S rRNA gene specific for SFB was amplified by PCR and normalized with amplification of the total bacterial (EUB) 16S rRNA gene. n=2 per group. Data are representative of two independent experiments. |
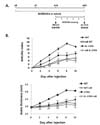 | Figure 2Treatment with antibiotics makes mice resistant to K/BxN serum-transferred arthritis. WT and IL-17-/- (IL-17KO) mice were fed with antibiotics (αB)- or vehicle-containing water from day 0 to day 24. The mice were injected i.p. with K/BxN serum on day 14 and sacrificed on day 24. (A) Experimental scheme. (B) Data combined from two separate experiments (total n=4-5 per group). Data are presented as means±SEM. Differences between groups were evaluated by the unpaired Student's t-test. p-values are reported for statistically significant differences between groups (<0.05). *p<0.05, **p<0.01, and ***p<0.001 when comparing WT with IL-17KO. ‡p<0.05, ‡‡p<0.01, and ‡‡‡P<0.001 when comparing WT with αB WT. |
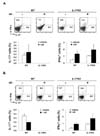 | Figure 3Profiles of cytokine-producing cells in Peyer's patches and mLNs from antibiotic-treated mice. WT and IL-17-/- mice that were treated with antibiotics (αB) or untreated were sacrificed 10 days after K/BxN serum transfer. Lymphocytes were extracted from the Peyer's patches (A) and mLNs (B) of those mice and assayed by intracellular FACS. Dot plots are representative of two mice and the percentage of cytokine-producing cells is expressed as the mean±SEM of each group. One representative result of two independent experiments is shown. |
 | Figure 4C3 deposition via the alternative complement pathway is decreased in the serum of antibiotic-treated mice. WT and IL-17-/- (IL-17KO) mice were fed with antibiotics (αB)- or vehicle-containing water for 14 days and injected i.p. with K/BxN serum. Serum was collected from individual mice 6 days after K/BxN serum transfer. An aliquot of mouse serum was incubated with zymosan particles, and C3 binding on the surface of zymosan was assayed by FACS. The histograms are representative of four mice per group. |
ACKNOWLEDGEMENTS
We thank Drs D. Mathis and Y. Iwakura for providing KRN mice and IL-17-/- mice, respectively. This work was supported by a National Research Foundation grant funded by the Korean Government (MEST; 2009-0081790).
References
2. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996; 87:811–822.

3. Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999; 10:451–461.

4. Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med. 2004; 10:40–45.

5. Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002; 196:77–85.

6. Jang E, Cho SH, Park H, Paik DJ, Kim JM, Youn J. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25- T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J Immunol. 2009; 182:4649–4656.

7. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012; 336:1268–1273.

8. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004; 4:478–485.

9. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008; 453:620–625.

10. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004; 303:1662–1665.

11. Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008; 180:559–568.

12. Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008; 4:337–349.

13. Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010; 32:815–827.

14. Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse model. Arthritis Rheum. 2006; 54:492–498.

15. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009; 139:485–498.

16. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31:331–341.

17. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010; 184:3336–3340.
18. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010; 464:1371–1375.

19. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009; 27:485–517.

20. Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011; 36:65–75.

21. Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci USA. 2010; 107:14292–14297.

22. Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001; 167:1601–1608.

23. Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009; 114:1005–1015.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download