Abstract
Collaboration of TLR and non-TLR pathways in innate immune cells, which acts in concert for the induction of inflammatory cytokines, can mount a specific adaptive immune response tailored to a pathogen. Here, we show that murine DC produced increased IL-23 and IL-6 when they were treated with LPS together with curdlan that activates TLR4 and dectin-1, respectively. We also found that the induction of the inflammatory cytokine production by LPS and curdlan requires activation of IKK. However, the same treatment did not induce DC to produce a sufficient amount of TGF-β. As a result, the conditioned media from DC treated with LPS and curdlan was not able to direct CD4+ T cells to Th17 cells. Addition of TGF-β but not IL-6 or IL-1β was able to promote IL-17 production from CD4+ T cells. Our results showed that although signaling mediated by LPS together with curdlan is a potent stimulator of DC to secrete many pro-inflammatory cytokines, TGF-β production is a limiting factor for promoting Th17 immunity.
The innate immune system detects infection via germ-line encoded receptors which recognize conserved microbial pathogen-associated molecular patterns (PAMP) such as lipopolysaccharide (LPS) and flagellin (1). Three main groups of pattern recognition receptors are the toll-like receptor (TLR), nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) and C-type lectin receptor (2). After recognition of PAMP, TLR recruit adaptors such as MyD88 and trigger downstream signaling cascades to activate nuclear factor-κB (NF-κB) and the mitogen-activated protein kinases (MAPK), which play a key role in inflammation and immune responses by regulating the expression of numerous inflammatory cytokine genes (3). C-type lectin receptors play essential roles in recognizing PAMP composed of carbohydrate residues. So far, one of the most studied C-type lectin receptors is dectin-1. Dectin-1, the receptor for β-glucan, was shown to be required for the control of fungal infection (4-6). Of particular interest is that dectin-1-activated dendritic cells (DC) instruct IL-17-producing T helper cells (Th17) differentiation (7). Dectin-1-activated DC secrete pro-inflammatory cytokines, including IL-6, TNF-α and IL-23, but little IL-12 (7).
Th17 cells have important roles in the pathogenesis of autoimmune disease (8). IL-23 was shown to be critically linked to autoimmunity after traditionally Th1-associated autoimmune disorders such as experimental autoimmune encephalitis (EAE) and collagen-induced arthritis (CIA) have been revisited (9). IL-23 is known to promote production of the pro-inflammatory cytokine IL-17 from CD4+ T cells (10). It has been shown that IL-23 is required for the terminal differentiation of Th17 cells in vivo (11). However, role of IL-23 for Th17 commitment is still controversial. Many groups have independently found that transforming growth factor-β (TGF-β) is necessary for the initiation of Th17 differentiation (9,12,13).
TGF-β is a pleiotropic cytokine with diverse biological activity regulating cell proliferation, differentiation, migration and survival (14,15). TGF-β is synthesized as a proprotein attached to the latency-associated peptide. Converting latent TGF-β to an active form is thus a critical regulation mechanism. TGF-β can be activated in vitro by transient acidification or protease treatment. TGF-β inhibits maturation of DC and also regulates antigen-presentation (14). Like many other cell types, DC also produce TGF-β but the function of DC-produced TGF-β remains unclear.
In this study, we investigated the effect of LPS and curdlan on the potential of DC to produce inflammatory cytokines. We found that simultaneous activation of TLR4 and Dectin-1 in DC increased production of pro-inflammatory cytokines including IL-23 and IL-6 greater than that by stimulation of each pathway alone. However, the same condition did not enhance the level of TGF-β, which resulted in poor Th17 differentiation. Therefore, to induce a potent Th17 cell mediated immune response, TGF-β must be provided by a different cell type or stimulation of DC through an additional pathway is likely required.
IL-10-deficient (Il10-/-) mice were purchased from the Jackson Laboratory. C57BL/6 mice were bred and maintained under specific pathogen-free conditions at the University of Michigan animal facility. All animal procedures were approved by the University of Michigan Committee on the Use and Care of Animals. Bone marrow-derived DC were prepared as previously described (16). MFB-F11 reporter cells were kindly provided by Dr. T. Wyss-Coray (Stanford University School of Medicine, Stanford, CA) (17).
LPS was obtained from Sigma-Aldrich. Curdlan was from Wako. CpG ODN 1668, lipoteichoic acid (LTA) and PolyI:C were purchased from InvivoGen. SB202190, BMS-345541, LY294002 and SP600125 were obtained from Calbiochem. U0126 was from Promega. Antibodies used for immunoblot analysis included p38 MAPK, phospho-p38 MAPK (Tyr180/Tyr182), p44/42 MAPK, phospho-p44/42 MAPK (Thr202/Tyr204), IKKβ, phospho-IKKβ (Ser180)/IKKβ (Ser181), SAPK/JNK, phospho-SAPK/JNK (Thr183/Tyr185), phospho-TAK1 (Thr184/187), IκBα (Cell Signaling Technology) and TAK1 (Santa Cruz Biotechnology). Neutralizing α-IL-10R antibody and isotype control rat IgG1 were purchased from BD Pharmingen.
Cytokine concentrations in supernatants were detected by ELISA and mRNA accumulation of cytokines was analyzed by qRT-PCR as previously described (16). TGF-β1 level was measured using DuoSet ELISA Development kit (R&D Systems). Briefly, 0.5 ml of culture supernatants were activated by adding 0.1 ml of 1 N HCl and neutralized with 0.1 ml of 1.2 N NaOH/0.5 M HEPES to detect total TGF-β1. The primers used for Gapdh, Il23a, Il12a, Il12b, Il10 and Il6 were described previously (18). The primers used for Il17a were 5'-GGACTCTCCACCGCAATGA-3' and 5'-GGCACTGAGCTTCCCAGATC-3', for Il17f were 5'-CCCCATGGGATTACAACAT CAC-3' and 5'-CATTGATGCAGCCTGAGTGTCT-3', for Il22 were 5'-CATGCAGGAGGTGG TACCTT-3' and 5'-CAGACGCAAGCATTTCTCAG-3', for Rorc were 5'-CCGCTGAGAGGG CTTCAC-3' and 5'-TGCAGGAGTAGGCCACATTACA-3', for Foxp3 were 5'-GGCCCTTCT CCAGGACAGA-3', 5'-GCTGATCATGGCTGGGTTGT-3', for Ifng were 5'-TCAAGTGGCAT AGATGTGGAAGAA-3' and 5'-TGGCTCTGCAGGATTT TCATG-3', for Tbx21 were 5'-ACAACCCCTTTGCCAAAGGA-3' and 5'-TCCCCCAAGCAGTTGACAGTT-3' and for Il4 were 5'-ACAGGAGAAGGGACGCCAT-3' and 5'-GAAGCCCTA CAGACGAGCTCA-3'.
Cells were lysed in lysis buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM DTT, 1% Triton X-100, 1 mM PMSF, 1% protease inhibitor cocktail (Sigma-Aldrich) and 25 µM MG132). Whole cell lysates were boiled in sample buffer (60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 5% β-mercaptoethanol and 0.1% bromophenol blue) and resolved by SDS-PAGE. After transfer to a polyvinylidene fluoride (PVDF) membrane (PerkinElmer), proteins were probed with the indicated antibodies and visualized by enhanced chemiluminescence (Thermo Fisher Scientific).
CD4+ T cells from C57BL/6 mice were purified using anti-CD4 magnetic beads (Miltenyi Biotec). Bone marrow-derived DC were stimulated with LPS (1 µg/ml), curdlan (100 µg/ml) or both for 24 h. DC culture supernatants (conditioned media (CM)) were filtered through 0.2 µm Pall Acrodisc syringe filters and kept at -80℃ before adding to the T cell culture. 1 ml of CD4+ T cell (5×105 cells/well) cultures were stimulated with plate-bound 5 µg/ml of anti-CD3 (2C11) and 1 µg/ml of anti-CD28 (37-51) in the presence of 2 ml of CM. Cultures were restimulated on day 5 with plate-bound anti-CD3. ELISA was performed after 48 h restimulation to detect IL-17A, IFN-γ and IL-4 production. mRNA levels were assessed by qRT-PCR after restimulation for 6 h. mRNA expression in freshly isolated CD4+ T cells was used as a control.
The generation of TGF-β reporting cell line (clone MFB-F11) and cell-based TGF-β bioassay have been described previously (17). MFB-F11 cells were maintained in D10 (DMEM supplemented with 10% FBS, 100 µg/ml penicillin, 100 µg/ml streptomycin) plus 125 µg/ml hygromycin. To determine bioactive TGF-β, cells were seeded in triplicate at 3×104 cells/well in 96-well flat-bottom plates in D10. After overnight incubation, cells were washed twice with PBS and incubated in 50 µl of serum-free DMEM media for 2 h. Conditioned media were added to the cells for 36 h. TGF-β1 (Peprotech) or serum-free DMEM was used as a positive or negative control, respectively. 12.5 µl of the culture supernatants were collected and SEAP activity was measured using Great EscApe SEAP Chemiluminescence Kit 2.0 (Clontech) according to the manufacturer's instructions.
Stimulation of DC via TLR or dectin-1 pathway was shown to elicit the preferential production of IL-12 or IL-23 respectively, which in turn directs Th1 or Th17 differentiation of CD4+ T cells (7). Because an immune system would encounter more than one component upon infection, we asked whether DC would exhibit a different potential to produce cytokines and subsequently induce a different T cell response when they are stimulated by both TLR ligand and dectin-1 agonist simultaneously. To do this, we treated DC with LPS, curdlan, or LPS and curdlan together (LPS+curdlan) and measured the level of cytokines in the supernatant. Treatment of DC with curdlan alone did not increase IL-23 production compared to LPS treatment (Fig. 1A left panel). IL-23 is a heterodimer consisting of p19 and p40 protein. mRNA expression of IL-23p19 and IL-12p40 during the course of curdlan treatment showed that the kinetics of IL-23p19 was gradual and slower than LPS treatment and the expression level of IL-12p40 was not increased (Fig. 1A middle and right panels). However, when DC were treated with LPS+curdlan, we observed that IL-23 production was substantially increased (Fig. 1B left panel). After co-treatment, mRNA expression of both IL-23p19 and IL-12p40 was steadily increased and prolonged but declined eventually (Fig. 1B middle and right panels). In addition, we found that IL-10 production was substantially increased after treating DC with LPS+curdlan (Fig. 1C left panel). Because IL-10 is a well-known anti-inflammatory cytokine, it is possible that IL-23 production could be suppressed by IL-10. If so, blocking IL-10 would further augment IL-23 induction. Indeed, IL-23 production was greatly enhanced when endogenous IL-10 was neutralized by adding the anti-IL-10 receptor antibody (α-IL-10R) but not the control antibody (Fig. 1C middle panel). DC from IL-10-deficient (Il10-/-) mice also showed a greater amount of IL-23 when they were stimulated with LPS+curdlan (Fig. 1C right panel). Together, co-stimulation of DC via TLR4 and dectin-1 pathway increased the production of inflammatory cytokines including IL-23 and IL-10.
We then asked which signaling pathways could contribute to the increased cytokine production after stimulation of DC with LPS+curdlan. Because LPS and curdlan activate signaling molecules including MAPK and IKK, we used pharmacological reagents to study the role of MAPK or IKK for the expression of cytokine genes. The gene expression of IL-6 and IL-10 was enhanced by treatment of LPS+curdlan (Fig. 2A). When cells were treated with BMS345541 that blocks IKKβ, IL-6 and IL-10 gene expression was totally abolished (Fig. 2A). In contrast, SP600125, SB202190 and U0126 which selectively inhibits JNK, p38 and MEK1/2, respectively, had relatively marginal effects on gene expression for both cytokines. Therefore, the results from the inhibitor study suggest that NF-κB but not MAPK signaling pathway plays a role during activation with LPS+curdlan. To substantiate this, we examined activation of signaling pathways by comparing the phosphorylation status of key MAPK and IKK signaling molecules by immunoblot analysis. We found that TAK1 that is an upstream kinase responsible for the activation of NF-κB and MAPK was activated by LPS but not curdlan. Treating DC with LPS+curdlan further increased TAK1 phosphorylation (Fig. 2B). To identify which downstream signaling pathway is activated by TAK1, we first assessed activation of IKK. IKKα/β was phosphorylated at the comparable level in DC treated with LPS alone or LPS+curdlan. However, IκBα degradation was faster in cells stimulated by LPS+curdlan supporting the activation of IKK. In contrast, we did not observe the similar increase in the phosphorylation status of p38, JNK and Erk1/2 by co-treatment of LPS and curdlan (Fig. 2B). Together, IKK seems to be a key regulator for the induction of inflammatory cytokines in DC upon the engagement of both TLR4 and dectin-1 signaling pathways.
IL-12 and IL-23 produced by DC are responsible for promoting Th1 and Th17 cells, respectively. We found that curdlan induced little production of IL-12p70 from DC and adding LPS+curdlan did not increase IL-12p70 (Supplementary Fig. 1A). This is likely due to the poor transcription of IL-12p35 gene by curdlan alone or together with LPS (Supplementary Fig. 1B). Therefore, treating DC with LPS+curdlan induces a set of inflammatory cytokines particularly those are known to be involved in Th17 cell differentiation.
We next investigated whether treatment of DC with LPS+curdlan promotes Th17 differentiation. To test this, DC were stimulated with LPS, curdlan, or LPS+curdlan and the supernatants (conditioned media; CM) were collected. Three different CM was then added to the CD4+ T cell cultures when they were stimulated with anti-CD3 and anti-CD28. Curdlan-treated CM induced IL-17A production more than LPS treatment (Fig. 3 left panel). Although LPS+curdlan treatment of DC induced a greater amount of IL-23 and IL-6 than LPS or curdlan alone (Fig. 1 and 2), IL-17A production was not enhanced by CM from LPS+curdlan treated DC (Fig. 3 left panel). When LPS or curdlan was added to the T cell culture directly, neither promoted IL-17A production suggesting that factors produced by DC are required (data not shown). Interestingly, curdlan-treated CM induced both IFN-γ and IL-4 production greater than LPS-treated CM, but adding CM from LPS+curdlan treated DC suppressed the induction of both cytokines (Fig. 3 middle and right panels). Therefore, even though DC produced inflammatory cytokines that would skew CD4+ T cells preferentially toward Th17 cells, CM from the same condition failed to enhance Th17 cell differentiation.
TGF-β together with IL-6, is considered necessary for IL-17 production from CD4+ T cells (13). Thus, it is possible that LPS+curdlan stimulated DC do not produce sufficient amounts of TGF-β, which then would result in a poor response of CD4+ T cells to make IL-17. To test this, we measured the levels of TGF-β produced by DC upon stimulation under the different conditions. As shown in Fig. 4A, the amount of total TGF-β1, measured by ELISA after transient acid treatment, showed that TGF-β1 was present in all conditions even without stimulation. Moreover, treating DC with LPS+curdlan did not increase the level of TGF-β1 over curdlan alone (Fig. 4A left panel). TGF-β was known to be produced as a latent form which binds to latency-associated peptide (LAP) (19). Hence, total TGF-β may not accurately represent its activity. To address this issue, we determined bioactive TGF-β in CM using a cell-based assay (17). Curdlan treated DC produced more active TGF-β than LPS treatment but the levels were comparable to LPS+curdlan treatment (Fig. 4A right panel). When we measured active TGF-β produced by DC, they produced approximately 5 pg (0.2 ng/ml) of TGF-β upon LPS+curdlan treatment (Supplementary Fig. 2). If the insufficient production of TGF-β caused poor Th17 differentiation, provision of exogenous TGF-β should generate a good response of CD4+ T cells. To test this, we added TGF-β together with CM to the CD4+ T cell cultures. Indeed, adding TGF-β but neither IL-6 nor IL-1β to CD4+ T cell cultures enhanced IL-17A production dramatically (Fig. 4B). The induction of IL-17 was accompanied by the increase of mRNA levels of IL-17 family cytokines, IL-17A, IL-17F and IL-22 (Fig. 4C). Moreover, RORγt and Foxp3, a key transcription factor for Th17 and regulatory T cell differentiation, respectively, were also elevated. However, Tbx21, a master transcription factor for Th1 cell differentiation, was not affected by TGF-β (Fig. 4C). Interestingly, TGF-β showed the suppressive effect on IFN-γ and IL-4 expression with CM from LPS+curdlan treatment but not curdlan alone (Fig. 4C). Together, our data demonstrated that treating DC with LPS+curdlan significantly induces the production of inflammatory cytokines but not TGF-β.
DC play an important role in priming naïve T cells by providing cytokines to mount an appropriate adaptive immune response tailored to a specific pathogen. For Th17 differentiation, TGF-β, IL-6 and IL-23 are essential to initiate and maintain IL-17 gene expression (11,13). Although DC are considered as a main source of these cytokines, the current study shows that DC do not produce a sufficient amount of TGF-β for Th17 cell differentiation. Therefore, it is not yet clear how TGF-β is provided. In particular, it remains elusive which cell type(s) could be a source of TGF-β for Th17 differentiation in vivo. Another possibility is that natural pathogens would trigger more than two pattern-recognition receptor pathways in DC. If so, activation of an additional signaling pathway might induce the expression of TGF-β or production of the biologically active form of TGF-β.
In conclusion, the present study demonstrated that collaboration of TLR4 and dectin-1 pathways in DC led to the increased production of inflammatory cytokines including IL-23 and IL-6 known to be important for Th17 cell differentiation. However, DC stimulated by LPS+curdlan were unable to promote IL-17 production from CD4+ T cells due to limited TGF-β.
Future studies are warranted to unveil the combined effects of the pattern-recognition receptors and their regulatory mechanisms in innate immune cells.
Figures and Tables
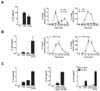 | Figure 1Co-stimulation of DC with LPS and curdlan increased IL-23 production. (A and B) BMDC prepared from C57BL/6 mice were stimulated with LPS (1 µg/ml), curdlan (100 µg/ml), or both. IL-23 was measured by ELISA at 24 h (left panels). mRNA levels of IL-23p19 (middle panels) and IL-12p40 (right panels) were determined at the indicated time by qRT-PCR. (C) IL-10 was measured by ELISA (left panel). IL-23 was measured by ELISA (middle panel). Cells were cotreated with LPS and curdlan in the presence of α-IL-10R (15 µg/ml) or rat IgG1 (15 µg/ml). BMDC prepared from C57BL/6 (Il10+/+) or Il10+/+ mice were treated with LPS, curdlan, or both and IL-23 was measured by ELISA (right panel). |
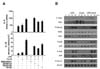 | Figure 2IKK plays an important role in cytokine expression for both LPS and curdlan treatment. (A) BMDC were pre-treated with indicated inhibitors (10 µM) for 30 min, followed by stimulation with LPS, curdlan, or both for 6 h. IL-6 and IL-10 mRNA levels were determined by qRT-PCR. (B) Cells were treated with LPS (1 µg/ml), curdlan (100 µg/ml), or both for the indicated time. P-TAK1, TAK1, P-IKKα/β, IKKβ, IκBα, P-p38, p38, P-JNK, JNK, P-Erk1/2 and Erk1/2 were detected by immunoblotting. |
 | Figure 3Co-treatment of DC with LPS and curdlan was unable to enhance Th17 differentiation. CD4+ T cells from C57BL/6 mice were stimulated with plate-bound anti-CD3 and anti-CD28 in the presence of conditioned media (CM) as described in the Materials and Methods. CM were prepared by treating BMDC with indicated stimulants for 24 h. T cell cultures were then restimulated on day 5 with plate-bound anti-CD3 for 48 h. ELISA was performed to detect IL-17A, IFN-γ and IL-4 production. *UT: CM from untreated DC. |
 | Figure 4Exogenous TGF-β but not IL-6 nor IL-1β enhanced IL-17 production in co-stimulated DC. (A) BMDC were stimulated with LPS (1 µg/ml), curdlan (100 µg/ml), or both. Total TGF-β1 and bioactive TGF-β were determined as described in the Materials and Methods. *Media: DC culture media (RPMI+5% serum); UT: CM from untreated DC. (B) CD4+ T cells were stimulated in the presence of CM plus indicated cytokines (IL-6 (40 ng/ml), IL-1β (+: 1 ng/ml, ++: 10 ng/ml), TGF-β (+: 2.5 ng/ml, ++: 5 ng/ml, +++: 25 ng/ml)). IL-17A was measured by ELISA after anti-CD3 restimulation. (C) CD4+ T cells were stimulated in the presence of CM with or without TGF-β (0.5 ng/ml). Cultures were restimulated for 6 h on day 5 with plate-bound anti-CD3 and mRNA levels were assessed by qRT-PCR. |
ACKNOWLEDGEMENTS
We thank Dr. Mark Kaplan (Indiana University, Indianapolis) for critical reading of the manuscript. This work was partly supported by NIH AI073677 to C.-H. Chang.
References
1. Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009; 227:221–233.

2. Hollmig ST, Ariizumi K, Cruz PD Jr. Recognition of non-self-polysaccharides by C-type lectin receptors dectin-1 and dectin-2. Glycobiology. 2009; 19:568–575.

4. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007; 8:31–38.

5. Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007; 8:39–46.

6. Carrion Sde J, Leal SM Jr, Ghannoum MA, Aimanianda V, Latge JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol. 2013; 191:2581–2588.
7. LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8:630–638.

8. O'Shea JJ, Steward-Tharp SM, Laurence A, Watford WT, Wei L, Adamson AS, Fan S. Signal transduction and Th17 cell differentiation. Microbes Infect. 2009; 11:599–611.
9. Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell line-age with regulatory T cell ties. Immunity. 2006; 24:677–688.

10. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007; 8:967–974.

11. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009; 10:314–324.

12. Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006; 441:231–234.

13. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006; 24:179–189.

14. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006; 24:99–146.
15. Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol. 2013; 191:3973–3979.
16. Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005; 201:1899–1903.

17. Tesseur I, Zou K, Berber E, Zhang H, Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC Cell Biol. 2006; 7:15.
18. Chang J, Voorhees TJ, Liu Y, Zhao Y, Chang CH. Interleukin-23 production in dendritic cells is negatively regulated by protein phosphatase 2A. Proc Natl Acad Sci USA. 2010; 107:8340–8345.

19. Taylor AW. Review of the activation of TGF-beta in immunity. J Leukoc Biol. 2009; 85:29–33.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download