INTRODUCTION
Sanguisorba officinalis L. (Rosaceae), a well known traditional medicinal plant in East Asia, is used for treatment of inflammatory and metabolic disease (1). Recent studies have been reported that various extracts of S. officinalis have medicinal effects such as anti-allergic, anti-cancer, anti-thrombin, anti-wrinkle, and neuroprotective properties (1,2,3,4,5,6,7). The root of S. officinalis includes various saponin components including triterpenoids and their glycosides, gallic acid, and disaccharide (8,9). ZYM-201 is a modified compound of ziyuglycoside, one of triterpene glycoside originated from the root of S. officinalis, through deglycosidation and esterification (10). Previous studies have shown that ZYM-201 plays a role in inhibiting tissue factor activity, tumor necrosis factor (TNF)-α production and modulating hyperlipidemic conditions (10,11,12).
LPS is the major component of the outer membrane of Gram-negative bacteria and commonly used to study inflammation because it initiates acute inflammatory responses in animals (13,14). The binding of LPS to Toll-like receptor 4 triggers signal transduction pathways and finally induces phosphorylation and translocation of nuclear factor (NF)-κB (15) leading to regulating the expression of a large number of genes which have important functions in immune responses (16). The stimulation of B cells by LPS induces proliferation and upregulates the expression of CD80 and CD86, which results in enhancing their antigen-presenting capacity (15,17,18,19). Increased expression of these costimulatory molecules is associated with activation of NF-κB (20,21).
Since ZYM-201 has been reported to inhibit the production of TNF-α, we investigated the effects of ZYM-201 on the activation of lymphocytes in LPS-induced inflammation. We found that the activation of LPS-stimulated B cells was abolished by ZYM-201 through inhibiting NF-κB activation and downregulating costimulatory molecules.
MATERIALS AND METHODS
Preparation of cells
All experiments were performed in accordance with the approval of Soongsil University Institutional Animal Care and Use Committee. 6-week-old C57BL/6 mice were purchased from Young Bio (Seongnam, Korea). To make cell suspensions, extracted spleens from mice were cut into small fragments and crushed between gauze. After depletion of red blood cells with Gey's solution, the cell suspensions were cultured in RPMI1640 (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin (GIBCO).
Chemicals
ZYM-201 is a methyl ester of triterpenoid glycoside and modified compound through deglycosidation and esterification from ziyu-glycoside, which is an originated component from the root of S. officinalis (10). Extraction, modification and purification of ZYM-201 were performed as previously described (10).
Flow cytometry
Splenocytes were cultured with 1 µg/ml LPS for 24 hours and/or ZYM-201 (50 or 100 µM) was added 6 hours after LPS treatment. mAbs for CD80 (clone 16-10A1), CD86 (clone GL1), and B220 (clone RA3-6B2) were purchased from eBioscience (San Diego, CA).
Western blot
Splenocytes were positively enriched by using FITC-conjugated B220 mAbs and anti-FITC microbeads according to the manufacturer's instructions (Miltenyi Biotec Ltd., Bergisch Gladbach, Germany). B220+ cells were cultured with 1 µg/ml LPS and/or 100 or 200 µM ZYM-201 for 30 minutes, and lysed in RIPA buffer (Sigma-Aldrich, St.Louis, MO, USA) supplemented with protease inhibitor cocktail tablets and PhosSTOP (Roche, Mannheim, Germany). Cell lysates were separated using SDS-PAGE and transferred on to polyvinylidene difluoride membrane (Millipore, Darmstadt, Germany). mAbs for HSP90 (clone C45G5), NF-κB p65 (clone L8F6), and phospho-NF-κB p65 (Ser536) (clone 93H1) were purchased from Cell Signaling Technology (Danvers, MA, USA). As the second step reagents, HRP-linked anti-rabbit IgG and anti-mouse IgG Abs were used (Cell Signaling Technology). Proteins were visualized by enhanced chemiluminescence (Bio-Rad, Hercules, CA, USA) and x-ray films (AGFA, Dusseldorf, Germany) were used for detection.
in vivo experiment
7-week-old C57BL/6 mice, weighing 22±2 g were used. Three groups were analyzed and there were four mice in each group. Mice in the control group were intraperitoneally injected with sterile PBS and mice in the other groups were intraperitoneally injected with 1 mg/kg LPS (Sigma-Aldrich) and then orally administrated with 20 mg/kg ZYM-201 dissolved in 0.5% carboxymethylcellulose (CMC) solution (Sigma-Aldrich) or with 0.5% CMC solution twice (1 and 7 hours after LPS injection). 24 hours after LPS injection, spleens were taken for analysis.
RESULTS AND DISCUSSION
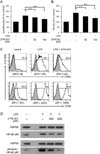
To investigate the effects of ZYM-201 on the cell number and activation of LPS-stimulated lymphocytes, cells from mouse spleens were cultured with 50, or 100 µM ZYM-201 after LPS treatment (Fig. 1A and 1B). The number of lymphocytes was increased from 100% (control) to 157% by LPS treatment, but decreased in the presence of ZYM-201 in a dose dependent manner: 142% with 50 µM ZYM-201 and 127% with 100 µM ZYM-201 (Fig. 1A). In particular, the number of B cells compared to other cell populations showed a big increase from 100% (control) to 185% by LPS treatment. However, this effect was abolished in the presence of ZYM-201; B cell numbers were decreased to 152% with 50 µM ZYM-201 and 132% with 100 µM ZYM-201 (Fig. 1B). In addition, the expression of CD80 and CD86 on LPS-stimulated B cells was down-regulated in the presence of ZYM-201; mean fluorescent intensity (MFI) of CD80 expression on LPS-stimulated B cells was downregulated from 330 to 222 followed by incubation with ZYM-201, and MFI of CD86 expression was also downregulated from 2410 to 1929 with ZYM-201 (Fig. 1C). These data indicate that ZYM-201 downregulates the activation of LPS-stimulated B cells. Because the upregulation of CD80 and CD86 costimulatory molecules is related with activation of NF-κB, we examined levels of NF-κB p65 phosphorylation on serine 536 (22) in B cells by western blot (Fig. 1D). Levels of NF-κB p65 phosphorylation in B cells were markedly increased by LPS stimulation, but dramatically decreased in the presence of ZYM-201 indicating that ZYM-201 has an inhibitory effect on LPS-stimulated B cell activation through inhibiting NF-κB phosphorylation and downregulating costimulatory molecules on B cells.
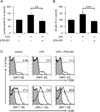
To confirm whether ZYM-201 has an inhibitory effect on LPS-stimulated B cell activation in vivo, cells from mice that had been injected with LPS and subsequently fed ZYM-201 twice were compared with those from mice that had not (Fig. 2). The numbers of lymphocytes and B cells were increased from 100% (control) to 138% and 143%, respectively, by LPS injection. Consistent with in vitro experiments, this effect was abolished after feeding ZYM-201; the numbers of lymphocytes and B cells were decreased to 92% and 90%, respectively, by ZYM-201 (Fig. 2A and 2B). Furthermore, the expression of constimulatory molecules on B cells in vivo showed the same pattern to that in vitro; the expression of CD80 and CD86 on LPS-stimulated B cells was downregulated after feeding ZYM-201 (Fig. 2C).
Taken together, these data indicate that ZYM-201, which has been known to have inhibiting tissue factor activity, TNF-α production and modulating hyperlipidemic conditions (10,11,12), has another function of anti-inflammatory activities via inhibition of NF-κB activation and downregulation of costimulatory molecules in B cells. In agreement with this, previous studies showed that inhibition of NF-κB activation suppress inflammatory responses (23) and have anti-inflammation and anticancer effects (24), and suggested that it could be used to treat immune diseases (25). Since ZYM-201 showed anti-inflammatory activities after LPS infection, we suggest that oral administration of ZYM-201 may be helpful to inflammatory diseases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download