Abstract
Understanding germinal center reactions is crucial not only for the design of effective vaccines against infectious agents and malignant cells but also for the development of therapeutic intervention for the treatment of antibody-mediated immune disorders. Recent advances in this field have revealed specialized subsets of T cells necessary for the control of B cell responses in the follicle. These cells include follicular regulatory T cells and Qa-1-restricted cluster of differentiation (CD)8+ regulatory T cells. In this review, we discuss the current knowledge related to the role of regulatory T cells in the B cell follicle.
Antibodies play an essential role in defending a host organism against infectious agents and mediate this host immunity through multiple mechanisms. Antibodies can neutralize viruses and prevent them from infecting host cells. In addition, they can inactivate toxins derived from infectious microorganisms. They can opsonize microorganisms and other foreign particles and thereby facilitate the recognition of these non-self-antigens by phagocytic cells through Fc receptors. Furthermore, antibodies can mediate the classical pathway of complement activation that can lead to formation of membrane attack complex which causes target cell lysis. In contrast to these beneficial roles, antibodies can also mediate detrimental effects to the host. For instance, binding of IgE expressed on the surface of mast cells to allergens can trigger local or systemic anaphylaxis. Moreover, formation of immune complexes as well as binding to self-antigens can induce the local or systemic inflammation that often occurs during autoimmune diseases (1). Therefore, humoral immunity is a double-edged sword that can either protect or damage host tissues, depending on the circumstances. Hence, the production of antibodies by B cells is subject to control by multiple positive and negative regulators.
Th2 cells have been recognized for decades as helper T cells that mediate B cell responses due to the essential role of IL-4 in promoting B cell proliferation and maturation (2,3,4). In addition to Th2 cells, a follicular helper T (Tfh) cell population has recently been discovered as a distinct lineage of helper T cells that is specialized for facilitating germinal center reactions (5,6,7). Tfh cells express CXCR5, which allows their migration into the B cell zone where they facilitate isotype switching and affinity maturation of Ig as well as the differentiation of B cells to plasma cells or memory B cells. Multiple types of immune cells have been identified as negative regulators of germinal center reactions. These include IL-10-producing B cells (8,9,10), Qa-1 restricted cluster of differentiation (CD)8+ regulatory T cells, and CXCR5+ forkhead box P3 (Foxp3)+ follicular regulatory T (Tfr) cells. The balance between positive and negative regulators of the germinal center reaction therefore determines the magnitude and kinetics of humoral immunity.
Most B cells reside in the B cell follicles of the secondary lymphoid organs, while some B cells travel throughout the body via the circulatory system. B cells in circulation can return to the B cell follicles by following a chemokine gradient of CXCL13 through the CXCR5 expressed on their surface, as dendritic cells in the follicles continuously produce CXCL13. The first step of antigen-specific humoral immunity is the binding of a cognate antigen to B cells through their surface immunogulobulin (B cell receptor). The binding of antigen to B cell receptors induces the migration of B cells toward the boundary between the B cell and T cell areas (11). Similarly, antigen-specific CD4+ T cells activated by dendritic cells transiently express CXCR5 on their surface, leading also to their migration toward the boundary between the B cell and T cell areas (Fig. 1). The migration of antigen-stimulated B and T cells to the same anatomic location increases the chances of antigen-specific interactions between B and T cells. This B:T cell interaction induces the initial proliferation of B cells. Some of these initially activated B cells further migrate toward the border of the T cell area (red pulp in the spleen) and form extrafollicular foci, where they can further differentiate into plasmablasts and short-lived plasma cells (Fig. 1). This type of extrafollicular B cell response is critical for the fast production of antibodies during infection (12). However, due to insufficient help from T cells, the affinity of such antibodies from extrafollicular B cell responses is relatively low.
Some of the B cells initially activated after the B:T interaction in the boundary between the B cell and T cell areas migrate back to the B follicles to form germinal centers. Germinal centers contain not only activated B cells but also follicular dendritic cells and Tfh cells. Follicular dendritic cells are stromal cells of non-hematopoietic origin. In addition to CXCL13, they express various types of Fc receptors as well as the complement receptors CR1 and CR2, allowing them to trap antigen-antibody-complement complexes for a long time without internalization. Hence, follicular dendritic cells are the main source of antigens that enable B cell proliferation and clonal selection in the germinal center (13). While initially activated CD4+ T cells transiently express CXCR5, some of them can be further differentiated into Tfh cells that stably express CXCR5 (14,15). Tfh cells can therefore migrate into B cell follicles following the CXCL13 gradient, just like B cells, where they further stimulate activated B cells, triggering isotype-switching, affinity maturation, and differentiation into long-lived plasma cells and memory B cells by providing cytokines such as IL-21 as well as costimulatory signals (Fig. 1). The follicular dendritic cells and Tfh cells are therefore indispensable for the generation of high-affinity antibodies and long-lived memory B cells.
Tfh cells localized to the germinal center of B cell follicles were first detected in the human tonsil. Further studies revealed that CXCR5 expression on these T cells is crucial for their migration into B cell follicles (16). However, these CXCR5+ T cells had been regarded as a subpopulation of Th2 cells for decades until recent studies clearly showed that CXCR5+ CD4+ T cells can be generated in mice lacking Th2 cells, such as STAT6-/- and IL-4-/- mice (17,18), indicating that CXCR5+ T cells are distinct from Th2 cells. Indeed, multiple groups have shown that the CXCR5+ CD4+ T cell population does not belong to Th1, Th2, or Th17 lineages but is instead a distinct subset of CD4+ T cells, termed follicular helper T cells (18). In addition to CXCR5, Tfh cells express ICOS, programmed death (PD)-1, B and T lymphocyte attenuator (BTLA) (in mice), CD40L, and CD84 (Fig. 2). Among them, detection of the CXCR5 marker in combination with either ICOS or PD-1 is the most widely used experimental method for identifying the Tfh cell population in mice as well as in humans.
While the surface phenotype of Tfh cells is well characterized, the development of the Tfh lineage from naïve CD4+ T cells is not completely understood. The transcriptional repressor Bcl-6 is necessary for the generation of Tfh cells, acting in part by repressing the transcription of Tbx21 (encoding T-box expressed in T cells [T-bet]) and Rorc (encoding retinoic acid-related orphan receptor γt [RORγt]) or direct binding to GATA-bind protein 3 (GATA3) (5,6,7). Bcl-6 was originally identified as a repressor of B lymphocyte-induced maturation protein-1 (Blimp-1), and expression of Blimp-1 is known to suppress the differentiation of Tfh cells. However, enforced expression of Bcl-6 alone in CD4+ T cells is not sufficient to drive Tfh cell differentiation since it cannot induce the expression of IL-21 and CXCR5 (16). Of note is a recent study by Liu et al where it was shown that the transcription factor achaete scutelike 2 (Ascl2) directly induces the transcription of CXCR5 in Tfh cells (19). In addition to Bcl-6 and Ascl-2, STAT3 (20,21,22), basic leucine zipper transcription factor (BATF) (23,24), and IFN regulatory factor 4 (IRF4) (25,26) are also known to be crucial for Tfh cell development. It is interesting to note that STAT3, BATF, and IRF4 are also needed for differentiation of the Th17 cell lineage.
Interestingly, a cluster of microRNAs known as miR17-92 has been reported to play a pivotal role during Tfh cell differentiation, although this role is still controversial. Initially the miR17-92 cluster was proposed to inhibit Tfh cell development (7); however, more recent studies have demonstrated that these microRNAs promote Th17 cells by facilitating the migration of Tfh cells into the B cell follicles through the suppression of the phosphatase pleckstrin homology domain leucine-rich repeat protein phosphatase 2, by suppressing the expression of Rora, or through both (27,28).
While the types of signal 3 for the differentiation of Th1, Th2, Th17, and induced regulatory T cells (Treg cells) are well demonstrated, it is still unclear which cytokine signals initiate the differentiation of Tfh cells. Due to its capacity to induce IL-21 expression in T cells, IL-6 has been proposed as a Tfh cell-inducing cytokine (20,29). By contrast, other studies have shown that Tfh cells are unaffected in mice with defective IL-6 signaling (21,30). Moreover, recent studies from multiple researchers convincingly showed that IL-12 can also drive Tfh cell differentiation by inducing IL-21 in a STAT3-dependent manner in mice as well as in humans (22,30,31,32). In this case, the balance between T-bet and Bcl-6 likely determines whether IL-12 stimulated CD4+ T cells differentiate into Th1 or Tfh cells. While the identity of the signal 3 cytokine for Tfh cells is unclear, negative regulation of Tfh cell development by IL-2 has been well established. Mechanistically, IL-2 induces the expression of Blimp-1 in a STAT5-dependent manner and therefore represses the expression of Bcl-6 (33,34). Therefore, the key transcription factors and cytokines driving the differentiation of Tfh cells are still obscure. Further studies will be needed to fully define the developmental regulation of the Tfh cell lineage.
Foxp3+ Treg cells are a subset of CD4+ T cells with immunosuppressive properties, including an ability to inhibit the proliferation and cytokine production of effector T cells (35,36). Notably, a series of recent studies have demonstrated that the suppression of each subset of helper T cell responses by Treg cells requires expression of a certain transcription factor together with Foxp3 in the Treg cells. For instance, Treg cells deficient in Bcl-6, T-bet, IRF4, or Stat3 failed to suppress Tfh, Th1, Th2, or Th17 cells, respectively (37,38,39,40,41,42) (Table I). Interestingly, these transcription factors are also indispensable for the differentiation of respective subsets of helper T cells. Thus, it seems likely that the differentiation of a particular Th subset and a corresponding Treg subset have similar developmental requirements. For instance, the expression of T-bet in conventional CD4+ T cells induces Th1 cells while its expression in Foxp3+ T cells induces Th1-specific Treg cells.
In humoral immune responses, Tfh cells are a unique subset of effector T cells capable of migration to the germinal center of B cell follicles. Uncontrolled Tfh responses trigger autoimmunity by inducing excessive autoantibodies (43). Similar to Tfh cells, Foxp3+ regulatory T cells coexpressing Bcl-6 gain the ability to migrate into B cell follicles through their surface-expressed CXCR5 (Fig. 1). These follicular regulatory T cells (Tfr) express CXCR5, ICOS, and PD-1, as well as Bcl-6, but lack CD40L, IL-4, and IL-21 (Fig. 2). They suppress germinal center reactions, including antibody affinity maturation and differentiation of plasma cells (38,40). These Tfr cells derive from CXCR5- natural Treg cells rather than naïve CD4+ T cells. Like Tfh cells, the differentiation of Tfr cells from CXCR5- Treg cells requires CD28 and ICOS costimulation as well as SAP-dependent stimulation by B cells (38,40).
Tfr cells in humans have also been described in studies of Treg cell migration in human lymphoid tissues. Most of the CD69- Treg cells in humans express CCR7 while only a small population of them express CXCR5. These CXCR5+ Treg cells in humans respond to a CXCL13 gradient in a chemotaxis assay and suppress antibody production by germinal center B cells and the function of Tfh cells in a coculture experiment in vitro (44,45). Thus, Tfr cells that are present in humans have an immunosuppressive capacity similar to that observed in murine Tfr cells.
Bcl-6+ Treg cells arise from natural Treg cells during active germinal center reactions (40). Since Bcl-6 is required for the expression of CXCR5 on Treg cells and CXCR5-deficient Treg cells are not able to suppress germinal center reactions, the capacity of Tfr to inhibit germinal center B and T cell responses depends on the expression of Bcl-6 in Treg cells (38,40). In addition, isolated Tfr cells have immunosuppressive properties that do not differ in their capacity to inhibit Tfh cells or other effector T cells in vitro. Indeed, the transcriptional profile of Tfr cells is similar to that of natural Treg cells with no distinct changes in suppressive function apart from that of chemotactic localization (38). Thus, the role of Bcl-6 in Treg cells might be the induction of CXCR5 expression on Tfr cells, thus promoting the capacity of Tfr cells to colocalize with activated B cells and Tfh cells in the germinal centers. Although Bcl-6 is known to repress the expression of Blimp-1, Tfr cell are shown to co-express Bcl-6 and Blimp-1 (40). Blimp-1 is known to mediate the expression of IL-10 in T cells (46,47). It will be interesting if Blimp-1-mediated production of IL-10 plays an important role in suppressing germinal center reactions by Tfr cells in vivo.
In addition to Bcl-6, signals from costimulatory molecules ICOS and CD28 are necessary for the development of Tfr cells (48). ICOS is shown to deliver early signals that induce the expression of Bcl-6 and CXCR5 in T cells (49). The phosphotidylinositol-3-kinase (PI3K) pathway has been reported to conduct the Bcl-6 induction signals further downstream of ICOS during Tfh cell differentiation (50).
Tissue necrosis factor receptor (TNFR)-associated factors (TRAFs) are cytoplasmic adaptor proteins and play a crucial role in the activation of both innate and adaptive immune cells. Molecules in the TRAF family are known to induce the activation of the NF-κB and MAPK pathways in response to various inflammatory stimuli, including TLR and IL-1R signaling (51). TRAF3 was the first identified TRAF with the ability to bind the cytoplasmic domain of the TNFR superfamily member CD40. TRAF3 exerts diverse functions depending on the type of receptor (51). In T cells, TRAF3 is crucial for TCR signaling (52). TCR/CD28 mediated early signaling includes phosphorylation of the ZAP70, linker for activation of T cells (LAT), phospholipase Cγ (PLC-γ), and ERK. T cells lacking TRAF3 showed impaired phosphorylation of these early signaling molecules, but exhibited normal TCR induced canonical NF-κB1 activation (52). Interestingly, we observed that mice with Treg-specific deletion of TRAF3 exhibited significantly increased IgG production upon immunization with sheep RBCs (53). These mice also showed increased numbers of Tfh cells and germinal center B cells, suggesting exaggerated germinal center reactions in the absence of TRAF3 in Treg cells. Mechanistically, the Tfr cell population significantly decreased in the Treg-specific TRAF3-deficient mice, indicating the crucial role of TRAF3 in the induction of Tfr cells. TRAF3 has little involvement in the induction of Bcl-6. Instead, TRAF3 seems to be required for the persistent expression of ICOS on Treg cells. Loss of TRAF3 in Treg cells resulted in decreased activation of the ERK-AP-1 signaling pathway upon the induction of TCR and CD28 signals. Thus, TRAF3 likely regulates ICOS gene induction on Tfr cells via the ERK-AP-1 signaling pathway (53), which is further supported by the fact that the ICOS promoter contains an AP-1 binding site (54). In addition, it has been shown that ERK signaling is essential for the induction of ICOS expression in T cells (55).
Treg cells regulate all aspects of the immune response. The mechanisms underlying Treg-mediated suppression include the production of suppressor cytokines (e.g., IL-10, TGF-β or IL-35), IL-2 consumption, granzyme-mediated cytolysis, and galectin-1-mediated suppression. In addition, Treg cells are also known to suppress the function of APC and indirectly inhibit the activation of effector T cells (56).
Surface expression of CTLA-4 and PD-1 on Treg cells is critical for controlling humoral immune responses, while the role of cytokines such as IL-10 or TGF-β is incompletely understood. CTLA-4 is an inhibitory molecule and exerts its inhibitory function by limiting the availability of CD80 and CD86 (57). Treg cell-specific deletion of CTLA-4 leads to the induction of fatal autoimmune and lymphoproliferative disorders with elevated levels of IgE and IgG in the serum and is also associated with the spontaneous development of Tfh cells and germinal center reactions (57).
PD-1 is another inhibitory receptor belonging to the same superfamily as CD28 and CTLA-4 (58). PD-1 is highly expressed on Tfh and Tfr cells in mice as well as in humans (38,59). PD-1-deficient mice showed dysregulated humoral immunity with increased produciton of autoantibodies (60,61), which is associated with increased expansion of Tfh cells (62). A recent study clearly showed the role of PD-1 in Tfr cells. In a co-transfer study, it was shown that Tfr cells lacking PD-1 exhibited increased expansion compared with wild-type Tfr cells. Moreover, PD-1-deficient Treg cells were more potent in suppressing antibody production from B cells (48), indicating that PD-1 suppresses the generation and function of Tfr cells in vivo. Thus it seems likely that PD-1 expression on Tfr cells places a regulatory check on Tfr cell activity, preventing the excessive suppression of germinal center reactions.
Although Foxp3+ Treg cells are known to produce IL-10 and TGF-β their role in the Tfr cell-mediated regulation of humoral immunity is unclear. The membrane-bound form of TGF-β was shown to mediate contact-dependent suppression of B-cell antibody production (63). IL-10 and TGF-β can induce isotype switching to IgG4 and IgA, respectively (64,65). Further studies will be needed to clarify the cellular and molecular mechanisms underlying Tfr cell-mediated suppression of germinal center reactions.
Although a subpopulation of CD8+ T cells had been long regarded as suppressor T cells, the CD8+ Treg cells have received far less attention since the discovery of Foxp3+ CD4+ regulatory T cells. This is at least in part due to the fact that both the identity and the mode of action of such CD8+ Treg cells have been obscure. However, recent advances in this field clearly showed that CD8+ Treg cells are a specialized subset of CD8+ T cells that can suppress activated CD4+ T cells in a Qa-1-dependent manner (66).
In addition to Foxp3+ T cells, Qa-1-reactive CD8+ T (CD8+ Treg) cells are known to suppress autoimmunity. Qa-1 is the murine homolog of a human class Ib MHC molecule, HLA-E. It can interact with the T cell receptors of CD8+ T cells, and thus mediates positive selection of Qa-1-reactive CD8+ T cells in the thymus (66). In addition, Qa-1 is also a ligand for natural killer group 2 (NKG2) molecules, including inhibitory CD94/NKG2A and CD94/NKG2C receptors on NK cells. Initial studies showed that Qa-1-deficient mice are more susceptible to experimental autoimmune encephalomyelitis, indicating the involvement of this molecule in peripheral tolerance (67,68,69). The Qa-1-dependent immunosuppression was found to be mediated by CD8+ T cells rather than in an NKG2-mediated manner. Similarly, some CD8+ T cells were also shown to suppress T cell-dependent B cell responses in a Qa-1-dependent manner in multiple animal models (70,71). Interestingly, the Qa-1 molecule is known to present autoantigens, including insulin and myelin basic protein. Therefore, it is likely that Qa-1-reactive CD8+ Treg cells are activated by recognizing self-antigens presented by Qa-1 and acquire suppressive activity against autoreactive T and B cells.
More recently, Cantor and colleagues elegantly showed the molecular and cellular mechanisms by which CD8+ Treg cells suppress adaptive immunity. They generated a Qa-1 knock-in mouse with a single amino acid exchange mutation. The mutated Qa-1 was found to be deficient in its ability to bind to T cell receptors and CD8, but maintained its binding to NKG2. Interestingly, these Qa-1 mutant mice exhibited significantly increased autoantibody levels against nuclear antigens as well as Ig deposition in renal glomeruli with increased frequency of Tfh cells in the secondary lymphoid organs (72). Of note, CXCR5+ CD4+ T cells were found to express Qa-1 on their surface while CXCR5- CD4+ T cells express very low levels of this non-classical MHC molecule, and therefore CD8+ Treg cells are able to specifically suppress Tfh cells by recognizing Qa-1 (72). Qa-1-reactive CD8+ Treg cells express ICOS ligand (ICOSL) and CD122, but unlike Tfr cells, they do not express ICOS and PD-1 (Fig. 2). In addition, the transcription factors driving the CD8+ Treg cell lineage have not yet been identified. Mechanistically, this Qa-1 mediated suppression of Tfh cells by CD8+ Treg cells is dependent on perforin. In summary, CD8+ Treg cells suppress Tfh cell-mediated production of autoantibodies by killing Tfh cells in a perforin-dependent manner upon recognizing Qa-1+ Tfh cells through their T cell receptors (72,73,74).
The immunoregulatory role of CD8+ Treg cells were also shown in murine models of autoimmune diseases, including the B6-Yaa mouse model of lupus and collagen induced arthritis (72,75). The IL-15/IL-15 receptor complex induces the expansion of CD8+ Treg cells, and transfer of the expanded CD8+ Treg cells was found to ameliorate the severity of autoimmune arthritis in an animal model by inhibiting autoantibody production (75).
It remains unclear whether Qa-1-reactive CD8+ Treg cells exist in humans. However, a few studies have suggested the existence of HLA-E-mediated immune suppression. For instance, the stimulation of CD8+ T cells with dendritic cells that were previously cultured with an HLA-E binding peptide can suppress self-reactive CD4+ T cells in patients with type 1 diabetes (76). Moreover, patients with multiple sclerosis exhibit reduced frequency of HLA-E-reactive CD8+ T cells in the peripheral blood (77). Nevertheless, whether the CD8+ Treg cells in humans play any role in Tfh responses remains unexplored. Further studies will be needed to demonstrate the role of these HLA-E-reactive CD8+ Treg cells in the regulation of autoimmune diseases in humans.
Production of high-affinity antibodies is a hallmark of a well-functioning host immune system. However, antibodies produced against self-antigens can destroy host tissues in a number of autoimmune diseases. Therefore, improved knowledge regarding the mechanisms responsible for the suppression of inappropriate antibody production has important implications for our understanding of the immunoregulatory control of autoimmunity as well as for the development of effective vaccines against infectious agents and malignancies. With respect to this aspect, it will be important to (i) delineate the underlying cellular and molecular mechanisms by which Tfr cells suppress germinal center reactions since it is not yet clear if they directly suppress B cells, Tfh cells, or both; (ii) determine whether adoptive transfer of Tfr cells can ameliorate ongoing autoimmune germinal center reactions in animal models of diseases; and (iii) determine if Tfr cells and CD8+ Treg cells can suppress autoimmunity in humans.
The use of regulatory T cells in the clinical setting has been largely unsuccessful. This might be due to the low frequencies of the specific subsets of Treg cells that are specialized for suppressing particular types of autoimmunity. For instance, the use of Tfr cells rather than a broader population of Treg cells could be more efficacious for the suppression of antibody-mediated autoimmunity, while the use of CXCR3+ Treg cells may better suppress unwanted Th1-mediated diseases (Table I). Thus, the identification of such a Treg cell subset could open new translational opportunities for the development of Treg cell-mediated therapies in the era of personalized medicine. In this case, the lower frequency of each Treg subset and the unknown stability of the Treg subset in vivo, as well as the poorly described antigen-specificity of the Treg cells are obvious obstacles. Further studies will be needed to further outline the basic biology of regulatory T cells as well as to demonstrate the translational potential of Tfr and CD8+ Treg cells before their use as novel therapeutics for antibody-mediated immune disorders in humans.
Figures and Tables
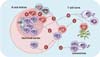 | Figure 1B cell responses to T-dependent antigens. (1) Naïve CD4+ T cells are activated by dendritic cells presenting cognate antigens in the T cell zones. They transiently express CXCR5 and migrate to the T-B border. B cells recognize their cognate antigens through surface Ig and migrate to the T-B border. In the T-B border, activated CD4+ T cells interact with B cells in an antigen-specific manner, termed "linked recognition." (2) Activated B cells then migrate either to B cell follicles or to the T cell zone. The latter B cells form extrafollicularly and differentiate into short-lived plasma cells with few somatic mutations. (3) Some of the activated CD4+ T cells can acquire characteristics of the Tfh cell lineage and stably express CXCR5. These Tfh cells migrate to the B cell follicle. (4) The activated B cells in B cell follicles are further stimulated by follicular dendritic cells and Tfh cells for multiple rounds. Follicular dendritic cells provide antigens to B cells, leading to the clonal selection of B cells that express high-affinity Ig on their surface. Tfh cells provide IL-21 and costimulation in order to induce the proliferation of B cells, isotype switching, and somatic mutation. The massive B cell expansion and differentiation lead to the formation of germinal centers in the follicle. (4') Tfr cells and CD8+ Treg cells are thought to suppress this germinal center reaction. (5) The germinal center reaction induces the differentiation of isotype-switched affinity-matured B cells into memory B cells or into long-lived plasma cells. |
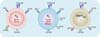 | Figure 2Comparison among Tfh, Tfr, and CD8+ Treg cells. Tfh cells express ICOS, PD-1, CD40L, and CXCR5 on the surface, as well as Bcl-6 and Ascl-2 in the nucleus. Similarly, Tfr cells express PD-1, ICOS, and CXCR5, but not CD40L. Unlike Tfh cells, Tfr cells express glucocorticoid-induced TNF receptor-related protein and CTLA4. They also express Foxp3, Bcl-6, and Blimp-1 in the nucleus. Qa-1-reactive CD8+ Treg cells express ICOSL, CD122, and CXCR5. The transcription factor required for the differentiation of this T cell subset remains to be determined. |
ACKNOWLEDGEMENTS
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University (YC), SNU invitation for distinguished scholar (YC), and the 2013 Yeungnam University Research Grant (JHC).
References
1. Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013; 382:819–831.

3. Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991; 251:70–72.

4. Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995; 268:720–722.

5. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009; 325:1006–1010.

6. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009; 325:1001–1005.

7. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009; 31:457–468.

8. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002; 3:944–950.

9. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002; 16:219–230.

10. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003; 197:489–501.

12. MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003; 194:8–18.

13. Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010; 237:72–89.

14. Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012; 8:337–347.

15. Fazilleau N, Mark L, Heyzer-Williams LJ, Heyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009; 30:324–335.

16. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011; 29:621–663.
17. King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009; 206:1001–1007.

18. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008; 29:138–149.

19. Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, Dong C. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014; 507:513–518.

20. Diehl SA, Schmidlin H, Nagasawa M, Blom B, Spits H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol Cell Biol. 2012; 90:802–811.

21. Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009; 113:2426–2433.

22. Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, Magdorf K, Kilic SS, Minegishi Y, Nonoyama S, French MA, Choo S, Smart JM, Peake J, Wong M, Gray P, Cook MC, Fulcher DA, Casanova JL, Deenick EK, Tangye SG. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012; 119:3997–4008.

23. Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, Kim CH, Taparowsky EJ. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med. 2010; 207:933–942.

24. Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, Murphy KM. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011; 12:536–543.

25. Biswas PS, Gupta S, Stirzaker RA, Kumar V, Jessberger R, Lu TT, Bhagat G, Pernis AB. Dual regulation of IRF4 function in T and B cells is required for the coordination of T-B cell interactions and the prevention of autoimmunity. J Exp Med. 2012; 209:581–596.

26. Bollig N, Brustle A, Kellner K, Ackermann W, Abass E, Raifer H, Camara B, Brendel C, Giel G, Bothur E, Huber M, Paul C, Elli A, Kroczek RA, Nurieva R, Dong C, Jacob R, Mak TW, Lohoff M. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc Natl Acad Sci U S A. 2012; 109:8664–8669.

27. Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, Bannard O, Bluestone JA, Matloubian M, Ansel KM, Jeker LT. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat Immunol. 2013; 14:840–848.

28. Kang SG, Liu WH, Lu P, Jin HY, Lim HW, Shepherd J, Fremgen D, Verdin E, Oldstone MB, Qi H, Teijaro JR, Xiao C. MicroRNAs of the miR-17 approximately 92 family are critical regulators of T(FH) differentiation. Nat Immunol. 2013; 14:849–857.

29. Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009; 206:69–78.

30. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011; 6:e17739.

31. Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, Schwartzberg PL, Hager GL, O'Shea JJ. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011; 35:919–931.

32. Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009; 31:158–169.

33. Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012; 36:847–856.

34. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012; 209:243–250.

35. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155:1151–1164.
36. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133:775–787.

37. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009; 326:986–991.

38. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011; 17:983–988.

39. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009; 10:595–602.

40. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011; 17:975–982.

41. Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009; 458:351–356.

42. Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009; 30:80–91.

43. Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009; 9:845–857.

44. Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004; 114:1640–1649.

45. Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005; 175:4180–4183.

46. Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kuhl AA, Heimesaat MM, Esser C, Im SH, Radbruch A, Rutz S, Scheffold A. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J Exp Med. 2014; 211:1807–1819.

47. Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011; 12:327–334.

48. Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013; 14:152–161.

49. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011; 34:932–946.

50. Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol. 2013; 25:366–372.

51. Hildebrand JM, Yi Z, Buchta CM, Poovassery J, Stunz LL, Bishop GA. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol Rev. 2011; 244:55–74.

52. Xie P, Kraus ZJ, Stunz LL, Liu Y, Bishop GA. TNF receptor-associated factor 3 is required for T cell-mediated immunity and TCR/CD28 signaling. J Immunol. 2011; 186:143–155.

53. Chang JH, Hu H, Jin J, Puebla-Osorio N, Xiao Y, Gilbert BE, Brink R, Ullrich SE, Sun SC. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med. 2014; 211:137–151.

54. Watanabe M, Nakajima S, Ohnuki K, Ogawa S, Yamashita M, Nakayama T, Murakami Y, Tanabe K, Abe R. AP-1 is involved in ICOS gene expression downstream of TCR/CD28 and cytokine receptor signaling. Eur J Immunol. 2012; 42:1850–1862.

55. Tan AH, Wong SC, Lam KP. Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J Biol Chem. 2006; 281:28666–28678.

56. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30:636–645.

57. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008; 322:271–275.

58. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006; 27:195–201.

59. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011; 17:975–982.

60. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003; 9:1477–1483.

61. Okazaki T, Otaka Y, Wang J, Hiai H, Takai T, Ravetch JV, Honjo T. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b-/-Pdcd1-/- mice. J Exp Med. 2005; 202:1643–1648.

62. Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010; 11:535–542.

63. Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001; 194:629–644.

64. Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998; 160:3555–3561.
65. Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989; 170:1039–1044.

66. Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004; 114:1218–1221.
67. Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004; 5:516–523.

68. Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007; 26:593–604.

69. Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008; 105:19420–19425.

70. Cantor H, Boyse EA. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The gen eration of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975; 141:1376–1389.

71. Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976; 143:1391–1340.

72. Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010; 467:328–332.

73. Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Semin Immunol. 2011; 23:446–452.

74. Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci U S A. 2011; 108:2010–2015.

75. Leavenworth JW, Tang X, Kim HJ, Wang X, Cantor H. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J Clin Invest. 2013; 123:1382–1389.

76. Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, Chess L. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010; 120:3641–3650.

77. Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008; 195:121–134.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download