Abstract
Chronic virus infection leads to the functional impairment of dendritic cells (DCs) as well as T cells, limiting the clinical usefulness of DC-based therapeutic vaccine against chronic virus infection. Meanwhile, B cells have been known to maintain the ability to differentiate plasma cells producing antibodies even during chronic virus infection. Previously, α-galactosylceramide (αGC) and cognate peptide-loaded B cells were comparable to DCs in priming peptide-specific CD8+ T cells as antigen presenting cells (APCs). Here, we investigated whether B cells activated by αGC can improve virus-specific T cell immune responses instead of DCs during chronic virus infection. We found that comparable to B cells isolated from naïve mice, chronic B cells isolated from chronically infected mice with lymphocytic choriomeningitis virus (LCMV) clone 13 (CL13) after αGC-loading could activate CD1d-restricted invariant natural killer T (iNKT) cells to produce effector cytokines and upregulate co-stimulatory molecules in both naïve and chronically infected mice. Similar to naïve B cells, chronic B cells efficiently primed LCMV glycoprotein (GP) 33-41-specific P14 CD8+ T cells in vivo, thereby allowing the proliferation of functional CD8+ T cells. Importantly, when αGC and cognate epitope-loaded chronic B cells were transferred into chronically infected mice, the mice showed a significant increase in the population of epitope-specific CD8+ T cells and the accelerated control of viremia. Therefore, our studies demonstrate that reciprocal activation between αGC-loaded chronic B cells and iNKT cells can strengthen virus-specific T cell immune responses, providing an effective regimen of autologous B cell-based therapeutic vaccine to treat chronic virus infection.
Lymphocytic choriomeningitis virus (LCMV) is usually used to study the relationship between virus infection and immune responses in the mice. When the mice are infected with LCMV clone 13 (CL13), one of the LCMV clones, the viruses are detected in the serum, spleen, and liver for more than a month and persist in the kidney and brain for more than 3 months (1). It has been well known that T cells exposed to persisting antigens differentiate into exhausted T cells rather than memory T cells (2,3,4), displaying a limited production of cytokines such as IFN-γ, TNF-α, and IL-2 as well as a upregulation of inhibitory receptors such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) on the cell surface (1,2,3,4). Interestingly, the generation of exhausted T cells also seems to be associated with functional impairment of dendritic cells (DCs), because during chronic virus infection DCs are known to limit the expression of major histocompatibility complex (MHC) and co-stimulatory molecules (3,5,6). When compared to the study of impaired function of DC during chronic virus infection, phenotype and function of B cells during chronic virus infection have not been fully understood. However, recent study has demonstrated that chronic virus infection redirected CD4+ T cell differentiation toward follicular helper T (Tfh) cells with the function of helping B cells, thereby sustaining virus-specific B cell responses (7). This observation suggests that B cells probably are intact during chronic virus infection, leaving room for the possibility of chronic B cells to become antigen presenting cells (APCs) when they are appropriately activated.
DC therapy as one of the useful ways in enhancing T cell responses has been applied for decades to treat cancer because DCs are the most specialized antigen presenting cells (APCs), which capture antigens, transport these antigens to lymphoid organs, and present them to T cells to improve immune responses (8). However, early DC therapy associated with the clinical trials has left tasks in terms of the standardization and quality control of technique such as evaluation of DC subsets, the optimal dose and frequency, route of administration and the optimal conditioning. Furthermore, there are still several problems in the efficient generation of DCs due to very few numbers of DCs in blood and lymphoid tissues, constraints of time and money for proliferation and differentiation ex vivo from blood monocytes, and immune attenuation of differentiated DCs (9,10,11).
It has been suggested that B cells have possibility to be applied as cell-based vaccine due to the ample quantity of B cells in the blood. In spite of relative abundance from blood and lymphoid tissues in comparison with DCs, B cells have been considered to be insufficient when introducing cell therapy due to low immune activity by derived from the deficiency of co-stimulatory molecules (11,12,13,14). However, when CD40 agonist was used as adjuvant, B cells could achieve immunogenicity and induce functional T cell responses in viral and tumor environment (15,16), suggesting that the possibility of B cells as alternative APCs in cell-based therapeutic intervention.
Most of CD1d-restricted invariant natural killer T (iNKT) cells that express invariant T cell receptor (TCR) α-chain (Vα14-Jα18 and Vα24-Jα18 in mice and humans, respectively) play a critical role in activation of B cells. iNKT cells represent unique T lymphocytes that co-express invariant TCR and NK cell marker, NK1.1, on their cell surface (17,18,19,20). To induce immunogenic B cells, α-galactosylceramide (αGC) known as a synthetic glycolipid has been used in many studies (21,22,23). Injection of αGC can activate T cells and NK cells and also develop the cellular immunity including innate immunity and CTLs responses (24,25). αGC has been known to combine with MHC class I-mediated CD1d expressed on surface of APCs, such as DCs and B cells, and then form CD1d-glycolipid complexes. These complexes are recognized by invariant TCR of iNKT cells (26). Interestingly, Chung et al. showed that iNKT cells are activated by αGC-loaded B cells and produce the effector cytokines such as IFN-γ and TNF-α. Subsequently, αGC-loaded B cells by activated iNKT cells were shown to up-regulate co-stimulatory molecules (CD40/86) and MHC class II in vivo (21). Moreover, B cells pulsed with ovalbumin (OVA) plus αGC could effectively induce the activation and proliferation of OVA-specific CD8+ T cells. As a results, it has been reported that interactions between αGC-loaded B cells and iNKT cells were able to inhibit and prevent cancer development (21,23,26).
In this study, we tested whether epitope-pulsed B cells isolated from chronically infected mice as well as naïve mice, when combined with αGC, can enhance antigen-specific CD8+ T cell responses in chronically infected mice and also contribute to the reduction of viremia. Such B cell therapeutic vaccine induced the expansion of virus-specific CD8+ T cells producing effector cytokine and lysosomal-associated membrane protein-1 (LAMP-1 or CD107a) and accelerated the control of viremia, suggesting a possibility of autologous B cell-based therapeutic vaccine to treat chronic virus infection.
5-week-old female mice (C57BL/6J) were purchased from Charles River Laboratories. P14 Thy1.1+ transgenic mouse were provided from Rafi Ahmed (Emory Vaccine Center, USA). Ly5.1+ congenic mice were provided from Dr. Myung-Ho Jang (POSTECH, Korea). All mice were maintained in a specific pathogen-free facility of the Yonsei Laboratory Animal Research Center (YLARC) of Yonsei University. All animal experiments were conducted in accordance with the International Animal Care and Use Committee (IACUC). For generation of chronic virus infection, C57BL/6J naive mice were infected via intravenous (i.v.) injection with 2×106 plaque-forming units (PFU) of LCMV CL13. In some experiments, CD4+ T cells were depleted via intraperitoneal (i.p.) injection with 250µg of mouse anti-CD4 antibody (GK1.5) one day early before infection of LCMV CL13. LCMV titers were determined by plaque assays, as described previously (27).
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation over Histopaque-1077 (density: 1.077 g/ml, Sigma-Aldrich) at 2,000 rpm for 20 min at 25℃. Lymphocytes were isolated from the spleens as previously described (28). The lungs were perfused with ice-cold phosphate-buffered saline (PBS) before removal for lymphocyte isolation. Lymphocytes from the lungs were isolated by using Percoll solution (Sigma-Aldrich) as previously described (27). B cells were separated from the spleen of naïve or LCMV CL13-infected mice using magnetic bead labeled with anti-CD19 antibody (Miltenyi Biotec). Purified B cells are >95% CD19 positive.
Flow cytometry was performed using FACS CANTO II (BD Biosciences). LCMV epitope-specific CD8+ T cells were detected by DbGP33-41 tetramer. Fluorescence-conjugated monoclonal antibodies were purchased from BD Biosciences, eBioscience, or Biolegend. To detect iNKT cells that express invariant TCR chain (Vα14-Jα18) in mice, iNKT cells were stained with CD1d tetramer, which was provided by NIH tetramer core facility, anti-TCR-β (H57-597), and anti-CD4 (RM4-5). For phenotypic analysis of donor B cells, splenocytes were stained with anti-CD19 (1D3), anti-CD40 (3/23), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD274 (MIH50), anti-CD273 (TY25), anti-CD80 (16-10A1), anti-CD86 (GL1), and anti-MHC class II (M5/114.15.2). Anti-Hamster IgG (229Arm), anti-Rat IgG2a (R35-95), anti-Rat IgG2b (A95-1), anti-Rat IgG1 (RTK2071), or anti-Mouse IgG1 (RTK2071) were used as isotype controls. For the measurement of T cell proliferation and activation, splenocytes were stained with anti-CD8 (53-6.7), anti-CD44 (IM7), anti-CD90.1 (OX-1), anti-CD107a (1D4B), anti-CD4 (RM4-5), anti-CD127 (A7R34), anti-CD62L (MEL-14), and anti-CD279 (29F.1A12). All the flow cytometry data was analyzed using the FlowJo software (Tree Star).
For detection of intracellular cytokines in T cells, lymphocytes isolated from the spleen and lung were stimulated in vitro with peptides including GP33-41 and GP276-286 (0.2µg/ml) in the presence of golgi-stop, golgi-plug, and anti-CD107a (1D4B) (BD Biosciences) for 5 h. Stimulated lymphocytes were permeabilized with Cytofix/Cytoperm (BD biosciences) and then stained with the following monoclonal antibodies (BD Biosciences): anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22), and anti-IL-2 (JES6-5H4).
αGC were provided by Chang-Yuil Kang's laboratory (Seoul University). Purified B cells were co-cultured with αGC (1µg/ml) for 18~20 h and then pulsed with GP33-41 peptide (1µg/ml) for 2 h in complete RPMI1640 medium. As a control group, vehicle (0.5% polysorbate) was used instead of αGC.
LCMV GP33-41-specific P14 CD8+ T cells were isolated from P14 transgenic mice using CD8+ isolation kit (Miltenyi Biotec). Purified P14 CD8+ T cells were labeled with CellTrace™ Violet (CTV) proliferation kit at concentration of 5µM (Invitrogen). Labeled P14 CD8+ T cells (1×107 cells) were adoptively transferred into naive mice.
Cross activation between αGC-loaded B cells and iNKT cells after transferring αGC-loaded B cells in vivo was previously demonstrated (23). We examined whether iNKT cells were efficiently activated by adoptively transferring αGC-loaded B cell isolated from chronically infected mice as well as from naive mice. LCMV CL13 infection into mice was proven to induce chronic viral infection but the virus titer in serum substantially decreases after a month (1). In comparison, when CD4+ T cells are depleted prior to infection with LCMV CL13, the serum virus has been known to persist throughout life. In the current study, we isolated chronic B cells from a long-term chronic infection model, which were generated after CD4+ T cell depletion, subsequent LCMV CL13 infection, and maintenance for more than 90 d post-infection (p.i.). B cells from a long-term chronic infection model (chronic B cells) as well as B cells from naive mice (naïve B cells) were cultured with αGC. After loading vehicle (veh)- or αGC-loaded naive and chronic B cells, the cells were adoptively transferred into naive congenic recipient (Fig. 1A). We first measured frequency of iNKT cells and their cytokine production in recipient mice to confirm the activation of iNKT cells by αGC-loaded B cells. Although the frequency of iNKT cells that were gated on CD4+ CD1d-tetramer+ TCRβinter was similar among groups, the production of effector cytokines (IFN-γ and TNF-α) from iNKT cells was significantly induced by adoptively transferring αGC-loaded B cells (Fig. 1B). More interestingly, comparable to αGC-loaded naïve B cells, αGC-loaded chronic B cells led to the production of IFN-γ and TNF-α from iNKT cells (Fig. 1B), indicating that chronic B cells as well as naïve B cells when loaded with αGC can efficiently activate iNKT cells in vivo.
We next determined whether αGC-loaded chronic B cells compared to naïve B cells can be reciprocally by the iNKT cells. Consistent with the previous reports (23), CD80, CD86, and MHC class II were upregulated on donor B cells within 24 h after injection of αGC-loaded B cells compared to veh-loaded B cells (Fig. 1C). In addition, MHC class I and PD-L1 were found to be upregulated after injection of αGC-loaded B cells compared to veh-loaded B cells. Of interest, the induction level of co-stimulatory (CD80 and CD86), co-inhibitory (PD-L1), and MHC class I/II molecules were comparable between αGC-loaded donor naive and chronic B cells (Fig. 1C). Since it has been already reported that iNKT cells activated by αGC-loaded naïve B cells contributed to the activation of αGC-loaded naïve B cells (21), the activation of αGC-loaded chronic B cells might be mediated by iNKT cells. This result demonstrate that chronic B cells as well as naive B cells can be activated in vivo by loading αGC.
Since the ultimate goal of therapeutic vaccination using autologous B cells is to treat chronic virus infection, it was required to test whether adoptive transfer of αGC-loaded chronic B cells can activate iNKT cells and be reciprocally activated by iNKT cells even in chronically infected mice. Very similar to Fig. 1A, we loaded αGC onto naïve and chronic B cells. These αGC-loaded B cells into chronically infected mice (over 90 d p.i.), which were initially depleted for CD4+ T cells and infected with LCMV CL13, instead of naïve mice (Fig. 2A). Frequency of iNKT cells was relatively low after transfer of αGC-loaded naïve and chronic B cells into chronically infected recipient mice compared to that of the B cells into naïve recipient mice (Fig. 2B). However, the ability of αGC-loaded naïve and chronic B cells to activate iNKT cells was still competent even in chronically infected mice (Fig. 2B). As well, the iNKT cells-mediated reciprocal activation of the B cells was also well observed in chronically infected recipient similar to naïve recipient mice (Fig. 2C compared to Fig. 1C). This result demonstrates that αGC-loaded chronic B cells can activate iNKT cells and be reciprocally activated by iNKT cells in chronically infected recipient similar to naïve recipient.
To investigate the capability of αGC-loaded chronic B cells to prime antigen-specific T cells in vivo, we examined the proliferation and function of LCMV epitope-specific CD8+ T cells after injection of αGC and LCMV epitope-loaded chronic B cells. P14 Thy1.1+ cells, which are CD8+ T cells expressing transgenic T cell receptor (TCR) specific to Db-restricted LCMV GP33-41 epitope, were labeled with CTV and transferred into naïve congenic mice. After 24 h, αGC-loaded and GP33-41 peptide (GP33)-pulsed naive or chronic B cells were injected into the recipient mice that were already transferred with P14 cells (Fig. 3A). Compared to naïve B cells loaded with veh or αGC, GP33-pulsed B cells showed a substantial proliferation of P14 cells (Fig. 3B). A portion of proliferating P14 cells produced effector cytokines such as TNF-α, IFN-γ, and IL-2 after in vitro restimulation with cognate peptide. In contrast, naïve B cells loaded with αGC and GP33 induced faster proliferation and better production of effector cytokines from proliferating P14 cells than cognate peptide only-pulsed naïve B cells without αGC (Fig. 3B). Similar to naïve B cells loaded with αGC and GP33, chronic B cells loaded with αGC and GP33 also led to a prominent proliferation of P14 cells and their production of effector cytokines (Fig. 3C) compared to cognate peptide only-pulsed chronic B cells. These results indicate that proliferation of epitope-specific CD8+ T cells and their cytokine production can be enhanced by the loading of αGC onto epitope-loaded chronic B cells as well as naïve B cells.
We next determined whether naïve B cells loaded with αGC and epitope can contribute to the control of viremia and the induction of antigen-specific CD8+ T cell responses during chronic viral infection. Twenty five days p.i. with LCMV CL13, when viral loads were between 103 and 104 PFU/ml of serum, the mice were therapeutically vaccinated with naïve B cells unloaded or loaded with αGC, GP33, or αGC plus GP33. This experimental setting allowed us to follow LCMV GP33-specific CD8+ T cell responses to the therapeutic B cell vaccine. We longitudinally monitored whether naïve B cells loaded with αGC plus GP33 can lead to the expansion of the GP33-specific CD8+ T cells in the blood of chronically infected mice. Loading of αGC alone on naïve B cells did not affect the frequency of GP33-specific CD8+ T cells, indicating that epitope loading onto αGC-activated B cells is essential to prime epitope-specific CD8+ T cells (Fig. 4A). Vaccination with naïve B cells loaded with αGC and GP33 showed a significant increase in the population of DbGP33-specific CD8+ T cells at 7 d post-vaccination compared to that with naïve B cells loaded with veh plus GP33, indicating in vivo effect of αGC-loading onto B cells (Fig. 4A). We compared fold-increase of the number of DbGP33-specific CD8+ T cells between 0 and 7 d post-vaccination, which showed that naïve B cells loaded with αGC and epitope increased the number of DbGP33-specific CD8+ T cells in maximum two-fold compared with any other groups (Fig. 4B). Mice receiving naïve B cells loaded with αGC and epitope showed accelerated decrease of virus from the blood compared to veh plus GP33 as well as veh or αGC alone post-vaccination (Fig. 4C). The reduction of viremia in the group vaccinated with αGC and epitope-loaded naïve B cells was the most significantly observed (p<0.01 compared to veh-loaded group) and better than that in the group vaccinated with epitope alone-loaded naïve B cells even though the difference was not significant (Fig. 4D). These results demonstrate that αGC loading enhanced the ability of B cells as APCs to prime epitope-specific CD8+ T cells and promoted the control of persisting virus during chronic infection.
To examine whether B cell therapeutic vaccination can induce LCMV-specific CD8+ T cell responses in tissues, we analyzed the frequency of population of DbGP33-specific CD8+ T cells in the spleen and lung at 2 wk post-vaccination. A significant increase of DbGP33-specific CD8+ T cells was observed in the group of B cells loaded with αGC and epitope compared with the group of veh-loaded B cells in the spleen (Fig. 5A). Also, in the lung as non-lymphoid tissue, we also observed a significantly higher frequency of DbGP33-specific CD8+ T cells in the group of αGC and epitope-loaded B cells than that in the other groups (Fig. 5B). To determine whether the virus-specific CD8+ T cells expanded after B cell vaccination sustain the cytolytic activity and produce the effector cytokine, we measured expression of CD107a, as a marker of degranulation, along with IFN-γ. Co-expression of IFN-γ and CD107a in CD8+ T cells after restimulation of GP276 peptide as well as GP33 peptide was the most significantly promoted after vaccination by B cells loaded with αGC and GP33 than that of other B cells (Fig. 5C and 5D; p<0.05 in GP33 response and p<0.001 in GP276 response compared to vaccination by veh-loaded B cells). It was worthwhile to note that CD8+ T cell response to GP276, which was not pulsed in B cells, was also enhanced after vaccination by αGC and GP33-loaded B cells. This suggests that B cells activated by αGC also induced endogenous LCMV-specific CD8+ T cells by presenting intrinsic antigen efficiently on αGC-activated B cells in vivo.
Through the previous experiment, we found that epitope-pulsed naïve B cells when activated appropriately with αGC could prime efficiently epitope-specific CD8+ T cells, leading to the reduction of viremia. To be useful for the treatment of persisting viremia, autologous B cells isolated from the same host that actually undergo chronic virus infection would be beneficial to prime epitope-specific CD8+ T cells due to MHC class I restriction to the corresponding epitope. Therefore, we explored to test whether the use of chronic B cells isolated from infection-matched mice can be applied into chronically infected mice as therapeutic B cell vaccine. B cells were purified from the mice chronically infected with LCMV CL13 (25 d p.i.), loaded with either veh plus GP33 or αGC plus GP33, and transferred into LCMV CL13 infection-matched recipient mice. After vaccination, the frequency of DbGP33-specific CD8+ T cells in the blood significantly increased by chronic B cells loaded with αGC and GP33 compared with those without αGC at 7 d after vaccination (Fig. 6A; p<0.05). In addition, the reduction of viremia was also clearly observed in the mice given the αGC and GP33-loaded chronic B cells at 14 d post-vaccination (Fig. 6B; p<0.05). Frequency of DbGP33-specific CD8+ T cells in the spleen was slightly higher in vaccination with αGC and GP33-loaded chronic B cells than in that with GP33 only-loaded chronic B cells (Fig. 6C) and the absolute numbers of DbGP33-specific CD8+ T cells in the spleen was also slightly higher even if the difference was not significant statistically (Fig. 6D; p=0.093). Of note, the frequency of functional CD8+ T cells (IFN-γ+ CD107a+ cells) responsive to GP33 or GP276 was higher in the mice given αGC and GP33-loaded chronic B cells than in those given GP33 only-loaded chronic B cells (Fig. 6E and 6F; p=0.054 for GP33 response and p=0.053 for GP276 response). It is also worthwhile to note that the percentage of dual producers for IFN-γ and CD107a among CD107a-expressing CD8+ T cells was higher in the group with αGC and GP33-loaded chronic B cells than in that with GP33 only-loaded chronic B cells (Fig. 6E). This observation suggests that αGC loading onto chronic B cells not only increase the number of epitope-specific CD8+ T cells but also improve their function per cell base. Taken together, these data firmly demonstrate that chronic B cells isolated from even chronically infected mice can be used as APCs to efficiently prime epitope-specific CD8+ T cells when they are optimally activated with αGC.
In this study, we focused on the possibility of αGC-activated autologous B cells as therapeutic B cell vaccine to treat chronic virus infection. We showed that B cells isolated from chronically infected mice could efficiently activate iNKT cells by αGC loading and reciprocally be activated by iNKT cells in chronically infected mice. Furthermore, the activated chronic B cells when pulsed with cognate epitope were able to fully activate epitope-specific CD8+ T cells. As therapeutic vaccination, αGC-loaded chronic B cells successfully enhanced antigen-specific CD8+ T cells and their function, resulting in accelerated control of viremia during chronic viral infection. These findings suggest that B cell-based cellular vaccine would provide a promising therapeutics in the control of persisting viruses.
Most dendritic cells (DC) based-vaccines have been clinically studied for treating cancer (9). However, DC therapeutic vaccination has not been applied to the treatment of chronic virus infection. It is probably caused that chronically infected DCs reinforced T cell exhaustion or tolerance rather than activation through interaction between inhibitory ligands such as PD-L1 and inhibitory receptors such as PD-1 (5). In addition, DCs during chronic virus infection were known to downregulate the expression of MHC and co-stimulatory molecules (3,5,6). To summarize, DC-based therapeutic vaccination is probably imperfect due to molecules inducing immunosuppression or immunological tolerance.
In this regard, we tested the usefulness of B cells instead of DCs as APCs to enhance CD8+ T cell responses during chronic virus infection. Through the previous studies, αGC-loaded B cells have been known to activate iNKT cells, which reciprocally convert αGC-loaded B cells from tolerogenic to immunogenic (21,26). Furthermore, immunogenic B cells pulsed with peptide and αGC generated therapeutic antitumor immunity (21). The most questionable concern was the status of B cells during chronic virus infection. Very surprisingly, unlike DCs during chronic virus infection, B cells isolated from chronically infected mice were almost comparable to naïve B cells in terms of their capability of iNKT cell activation, their reciprocal activation by iNKT cells, and their activity to prime epitope-specific CD8+ T cells. There might be explanation for a relative intactness of B cells compared to DCs. Firstly, some viruses including LCMV were highly produced by adherent cells (~1%), such as dendritic cells and macrophages, compared with B cells (≤0.01%), which is based on the results from the splenocytes isolated from carrier mice infected with LCMV Armstrong strain at birth (29). This situation appeared to minimize the virus infection-mediated downregulation of MHC and co-stimulatory molecules in B cells. Secondly, CD4+ T cells were reported to be essential in the maintenance of viremia control via producing interleukin-21 (IL-21) during persistent infection (30,31,32). Fahey et al. also previously reported that chronic virus infection did not hamper the ability of CD4+ T cells to help B cells (7). LCMV-specific CD4+ T cells can be differentiated into IL-21-producing follicular helper T cells. These Tfh cells are essential for germinal center (GC) reaction to produce the high-affinity antibody secreting plasma cells as well as memory B cells (7). IL-21 affects directly on B cells to develop GC and to generate antibody production (33,34).
However, the above reports did not examine the phenotype of B cells during chronic virus infection. When we directly compared the phenotype of B cells from naïve and chronically infected mice, the expression levels of co-stimulatory molecules such as CD40, CD80, and CD86 as well as MHC class I/II molecules, indeed, were very similar between two different B cells (data not shown). It is probably the cause of no difference in B cell-based vaccine efficacy using different source of B cells. This observation together with our data suggests that B cell function as APCs probably is intact during chronic virus infection and thus, autologous B cells isolated from chronically infected host, when appropriately activated, could be used as a beneficial source of therapeutic cell-based vaccine to treat chronic virus infection.
It was very interesting finding that PD-L1, one of the coinhibitory molecule, as well as co-stimulatory molecules was also highly upregulated on reciprocally activated donor B cells in the mice given αGC-loaded B cell vaccine, as shown in Fig. 1C and 2C. Since PD-1, the receptor for PD-L1, is highly expressed on exhausted CD8+ T cells during chronic virus infection (28,35,36), the interaction of PD-1 on exhausted CD8+ T cells with PD-L1 on αGC-activated B cells might inhibit TCR signal transduction, cytokine production, and cytotoxicity. Ha et al. has reported that exhausted CD8+ T cells could overcome immune tolerance by blocking PD-1, thereby enhancing function of APCs and restoring the function of exhausted CD8+ T cells (27). Therefore, using a combination of αGC-loaded B cells and blocking PD-1 may synergistically enhance CD8+ T cell responses and accelerate viral control.
In future, more researches have to be done to clinically use B cells as source of cell-based vaccine. Although αGC can induce immunogenic B cells, DCs are still better inducer of immunogenicity than B cells. In addition, compared to DCs, a large number of B cells are needed for vaccination. There is a report showing that B cells can be expanded ex vivo by co-culturing with CD40 stimulator cells in the medium containing IL-4 (37). Therefore, to improve the efficacy of B cell-based vaccine, the technologies to enhance B cell immunogenicity, to optimally cross-present antigens to MHC class I on B cells, and to multiply B cells in vitro should be devised. If the improvement is fulfilled, cellular vaccine strategy using B cells as APCs would offer a promising tool for immunotherapy against persisting pathogens.
Figures and Tables
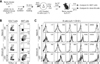 | Figure 1Comparison of αGC-loaded naïve or chronic B cells for bidirectional activation of iNKT and B cells in naïve mice. naïve and chronic B cells were isolated from splenocytes of naïve mice and chronically infected mice that were initially depleted of CD4+ T cells and subsequently infected with LCMV CL13 (over 90 d p.i.), respectively. (A) Schedule for αGC-loading onto B cells and generation of in vivo activated iNKT cells and B cells. Naive and chronic B cells isolated from Ly5.1+ naïve and chronically infected mice were cultured with vehicle (veh) or αGC in vitro for 18~20 h. 2×106 cells of αGC-loaded B cells were adoptively transferred into Ly5.2+ congenic naive mice. The recipient mice were sacrificed 6 and 24 h after adoptive transfer of B cells for analysis of the activation of iNKT cells and donor B cells, respectively. (B) In vivo activation of iNKT cells by αGC-loaded naïve and chronic B cells in naïve mice. Frequency of iNKT cells among CD4+ T cells was examined by staining with CD1d tetramer (tet) and TCR-β (CD1d tet+ TCR-βinter) and their function was evaluated by intracellular staining of IFN-γ and TNF-α. The number in the plot indicates the percent of corresponding population. (C) In vivo activation of donor αGC-loaded B cells by activated iNKT cells in naïve mice. Ly5.1+ CD19+ donor B cells were gated and analyzed for the expression of surface molecules. The number in histogram plot represents mean fluorescence intensity (MFI) of the expressed protein. The vertical grey line in histogram plot indicate a geometric mean level of the protein expressed on naïve B cells loaded with vehicle. Results are representative of at least three independent experiments. |
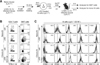 | Figure 2Comparison of αGC-loaded naïve or chronic B cells for bidirectional activation of iNKT and B cells in chronically infected mice. Naïve and chronic B cells were isolated from splenocytes of naïve mice and chronically infected mice that were initially depleted of CD4+ T cells and subsequently infected with LCMV CL13 (over 90 d p.i.), respectively. (A) Schedule for αGC-loading onto B cells and generation of in vivo activated iNKT cells and B cells. Naive and chronic B cells isolated from Ly5.1+ naïve and chronically infected mice were cultured with veh or αGC in vitro for 18~20 h. 2×106 cells of αGC-loaded B cells were adoptively transferred into Ly5.2+ congenic mice that were already infected with LCMV CL13 (over 90 d p.i.). The recipient mice were sacrificed 6 and 24 h after adoptive transfer of B cells for analysis of the activation of iNKT cells and donor B cells, respectively. (B) In vivo activation of iNKT cells by αGC-loaded naïve and chronic B cells in chronically infected mice. Frequency of iNKT cells among CD4+ T cells was examined by staining with CD1d tet and TCR-β (CD1d tet+ TCR-βinter) and their function was evaluated by intracellular staining of IFN-γ and TNF-α. The number in the plot indicates the percent of corresponding population. (C) In vivo activation of donor αGC-loaded B cells by activated iNKT cells in chronically infected mice. Ly5.1+ CD19+ donor B cells were gated and analyzed for the expression of surface molecules. The number in histogram plot represents mean fluorescence intensity (MFI) of the expressed protein. The vertical grey line in histogram plot indicate a geometric mean level of the protein expressed on naïve B cells loaded with vehicle. Results are representative of at least three independent experiments. |
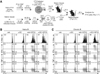 | Figure 3
In vivo priming of antigen-specific CD8+ T cells by αGC and epitope-loaded naïve and chronic B cells. Naïve and chronic B cells were isolated from splenocytes of naïve mice and chronically infected mice that were initially depleted of CD4+ T cells and subsequently infected with LCMV CL13 (over 90 d p.i.), respectively. Naïve P14 CD8+ T cells were purified from splenocytes of naïve P14 mice. (A) Schedule for analysis for in vivo activity of αGC and peptide-loaded naïve and chronic B cells to prime antigen-specific CD8+ T cells. 5×106 of CellTrace™ Violet (CTV)-labeled P14 Thy1.1+ CD8+ T cells were adoptively transferred into Thy1.2+ congenic naïve mice. After 24 h, the mice were given with 1×106 of naïve or chronic B cells loaded with veh, αGC, veh plus GP33-41 peptide (GP33), or αGC plus GP33. The recipient mice were sacrificed 48 h after adoptive transfer of B cells for analysis of donor P14 cells. (B and C) Proliferation (1st row) and cytokine production (2nd to 4th row) of P14 cells primed with αGC plus GP33-loaded naïve (B) and chronic B cells (C). Donor Thy1.1+ CD8+ T cells in the spleen were gated and examined for CTV dilution along with cytokine production. Division time and frequency of dividing cell population are indicated in histogram plot. The number as shown in each quadrant of the plot represents percentage of the corresponding cell population. Results are representative of at least three independent experiments. |
 | Figure 4Effect of therapeutic vaccination with αGC and epitope-loaded naïve B cells on T cell responses and virus control during chronic virus infection. LCMV CL13-infected mice were vaccinated with 1×107 of veh, αGC, veh plus GP33, or αGC plus GP33-loaded naïve B cells at 25 d p.i. (A) Frequency of DbGP33-41 tet-positive cells among CD8+ T cells in the chronically infected mice after therapeutic vaccination with B cells. At 0, 7, and 14 d post-vaccination (25, 32, and 39 d p.i.), CD8+ T cells in the blood were gated and evaluated for the expansion of DbGP33-41 tet+ cells. (B) Fold-increase of DbGP33-41 tetramer specific CD8+ T cells in PBMCs between 0 and 7 d post-vaccination. (C) Change of virus titer in the blood post-vaccination. Serum virus titer was determined from individual mice before (17 d p.i.) and after therapeutic vaccination with B cells (39 d p.i.). Vertical line in the graph indicates time point when therapeutic vaccination was performed. (D) Reduction of serum virus titer after B cell therapeutic vaccination. Fold-decrease of serum virus titer was calculated by serum virus titer before therapeutic vaccination (17 d p.i.) divided by that after therapeutic vaccination with B cells (39 d p.i.). Results are representative of two independent experiments. n=4 mice per group in each experiment. ns, not significant; *p<0.05; **p<0.01. |
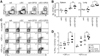 | Figure 5Expansion of epitope-specific CD8+ T cells and their effector function after therapeutic vaccination with αGC and epitope-loaded naïve B cells. LCMV CL13-infected mice were vaccinated with 1×107 of veh, αGC, veh plus GP33, or αGC plus GP33-loaded naïve B cells at 25 d p.i. and sacrificed at 39 d p.i. (14 d post-vaccination). (A) Frequency of DbGP33-41 tet+ cells among CD8+ T cells in the spleen. (B) Total number of DbGP33-41 tet+ cells in the spleen and lung. (C) IFN-γ and CD107a expression in CD8+ T cells in the spleen after in vitro stimulation with GP33-41 or GP276-286 peptide. (D) Proportion of CD8+ T cells producing IFN-γ and CD107a in the spleen after peptide stimulation. Number in the plot indicates percentage of the corresponding cells. Results are representative of two independent experiments. n=3 mice per group in each experiment. ns, not significant; *p<0.05; ***p<0.001. |
 | Figure 6Therapeutic efficacy of αGC and epitope-loaded chronic B cells during chronic virus infection. Four different groups of B cells were purified from LCMV CL13-infected mice (25 d p.i.) and treated with veh plus GP33 or αGC plus GP33. LCMV CL13-infected mice were vaccinated at 25 d p.i. with 1×107 of each group of B cells as therapeutic vaccine and sacrificed at 39 d p.i. (14 d post-vaccination). (A) Fold-increase of DbGP33-41 tetramer specific CD8+ T cells in PBMCs between 0 and 7 d post-vaccination (between 25 and 32 d p.i.),. (B) Reduction of serum virus titer after B cell therapeutic vaccination. Fold-decrease of serum virus titer was calculated by serum virus titer before therapeutic vaccination (17 d p.i.) divided by that after therapeutic vaccination with B cells (39 d p.i.). (C) Frequency of DbGP33-41 tet+ cells among CD8+ T cells in the spleen. (D) Total number of DbGP33-41 tet+ cells in the spleen. (E) IFN-γ and CD107a expression in CD8+ T cells in the spleen after in vitro stimulation with GP33-41 or GP276-286 peptide. (F) Proportion of CD8+ T cells producing IFN-γ and CD107a in the spleen after peptide stimulation. Number in the plot indicates percentage of the corresponding cells. Results are representative of two independent experiments. n=3 mice per group in each experiment. ns, not significant. |
ACKNOWLEDGEMENTS
This work was supported by the Basic Science and Research Program (2013R1A1A2010389) and Bio & Medical Technology Development Program (2012M3A9B4028264) of the National Research Foundation of Korea (NRF) funded by the Korea government (MEST). This work was also supported by a grant of the Mogam Biotechnology Institute (2011-8-1995).
References
1. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003; 77:4911–4927.

2. Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008; 223:317–333.

4. Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998; 188:2205–2213.

5. Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011; 44:217–231.

6. Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013; 13:652–664.

7. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011; 208:987–999.

8. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004; 10:909–915.

9. Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic Cell Immunotherapy: Mapping The Way. Nat Med. 2004; 10:475–480.

10. Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010; 2:57–68.

11. Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004; 25:659–664.

12. Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992; 175:131–138.

13. Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992; 258:1156–1159.

14. Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997; 100:2757–2765.

15. Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004; 103:2046–2054.

16. Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003; 63:2836–2843.
19. Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005; 23:877–900.

20. Park SH, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000; 406:788–792.

21. Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, Kang CY. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006; 66:6843–6850.

22. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004; 114:1379–1388.

23. Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM, Chung Y, Kang CY. alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer. 2008; 122:2774–2783.

24. Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003; 171:5140–5147.

25. Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003; 170:2540–2548.

26. Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009; 9:28–38.

27. Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008; 205:543–555.

28. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006; 439:682–687.

29. King CC, de Fries R, Kolhekar SR, Ahmed R. In vivo selection of lymphocyte-tropic and macrophage-tropic variants of lymphocytic choriomeningitis virus during persistent infection. J Virol. 1990; 64:5611–5616.

30. Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009; 324:1569–1572.

31. Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009; 324:1576–1580.

32. Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009; 324:1572–1576.

33. Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010; 207:353–363.

34. Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010; 207:365–378.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download