Abstract
IL-33 is a member of the IL-1 cytokine family and plays a role in the host defense against bacteria, viruses, and fungi. In this study, we investigated the function of IL-33 and its receptor in in vitro macrophage responses to Candida albicans. Our results demonstrate that pre-sensitization of isolated peritoneal macrophages with IL-33 enhanced their pro-inflammatory cytokine production and phagocytic activity in response to C. albicans. These macrophage activities were entirely dependent on the ST2-MyD88 signaling pathway. In addition, pre-sensitization with IL-33 also increased ROS production and the subsequent killing ability of macrophages following C. albicans challenge. These results indicate that IL-33 may increase anti-fungal activity against Candida through macrophage-mediated resistance mechanisms.
IL-33 is a multifaceted, multifunctional cytokine. It was initially found to be highly expressed in the nuclei of endothelial and epithelial cells (1). Binding of IL-33 to a heterodimeric receptor complex consisting of ST2 and IL-1RAP results in the recruitment of an adapter protein, MyD88, and the onset of signal transduction (2). IL-33 signaling induces the production of various mediators involved in inflammation and tissue repair by nonhematopoietic and hematopoietic cells, including endothelial cells, epithelial cells, macrophages, basophils, eosinophils, and mast cells (3).
Candida albicans is a commensal organism of the gastrointestinal tract and vagina (4); however, it is also the most common pathogen associated with mucosal and systemic infections. Invasive candidiasis is the fourth leading cause of nosocomial bloodstream infection in the USA (5), and it is estimated to occur worldwide in over 400,000 people every year with mortalities ranging from 46% to 75% despite administration of antifungal therapy in modern intensive care unit facilities (6). The resistance mechanism of mice to C. albicans infection relies mainly on the phagocytic and killing ability of innate immune cells, such as neutrophils and macrophages (7,8). Our previous studies have shown that IL-33 enhances neutrophil recruitment to site of infection and their phagocytic activities in peritoneal C. albicans infection (9,10). Little is known about the role of IL-33 in macrophages during Candida infection. In this study, we demonstrate that pre-sensitizing peritoneal macrophages with IL-33 enhances various activities that facilitate fungal clearance.
C57BL/6 mice were purchased from Orient Bio-Charles River. MyD88 KO mice with a C57BL/6 genetic background were maintained in a specific pathogen-free facility and used when they were 7 to 8 weeks old. All experiments were conducted according to the regulations of the Animal Committee of the University of Ulsan.
Mice were intraperitoneally injected with 3 ml of sterile 3% thioglycollate broth (Difco). After 3 days, mice were sacrificed and peritoneal exudate cells (PECs) were harvested from the peritoneal cavities. The cell pellet was washed and resuspended in DMEM medium supplemented with 10% fetal bovine serum (FBS). PECs were seeded into 24-well tissue culture plate (1×106 cells/ml) and incubated at 37℃ for 2 h, at which point the adherent cells were harvested.
Cytokines present in culture supernatants were measured by ELISA (eBioscience or R&D systems), according to manufacturers' protocols.
Total RNA was extracted from isolated peritoneal macrophages using TRIzol (Invitrogen), according to the manufacturer's manufacturer's instructions. cDNA was synthesized from 2µg of total RNA using SuperScript reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR Green PCR Master Mix (Qiagen) in the ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The primers used to measure levels of ST2 mRNA were 5'-TGA CGG CCA CCA GAT CAT TCA CAG-3' (forward) and 5'-GCC AAA GCA AGC TGA ACA GGC AAT AC-3' (reverse).
In vitro phagocytosis assays were performed as previously described (7). Briefly, peritoneal macrophages were incubated with IL-33 (100 ng/ml) at 37℃ for 2 h. Heat-killed C. albicans was labeled with FITC, opsonized, and challenged against IL-33-primed macrophages at 37℃ for 20 min (MOI=10). Phagocytosis was stopped by transferring cells to ice and washing thoroughly with cold FACS buffer. Extracellular fluorescence was quenched by adding 200µl of PBS containing 0.04% trypan blue and 1% formaldehyde. Fungus-containing cells were then counted by flow cytometry and the degree of phagocytosis expressed as the percentage of FITC-positive macrophages.
Macrophages were mixed with opsonized live C. albicans (MOI=1) and incubated at 37℃ for 20 min with continuous rotation. Cells were washed thoroughly in cold PBS, resuspended in warm DMEM medium, and further incubated at 37℃. At the indicated times, 200 ml samples were harvested and cells lysed in PBS containing 0.1% Triton X-100. CFUs were quantified by plating lysates on agar. Percent killing was calculated as [1-(CFUs after incubation/phagocytosed CFUs at the start of incubation)]×100.
Macrophages were pre-sensitized with IL-33 (100 ng/ml) or PBS for 2 h and were then further incubated in the presence of 2µM 2',7'-dichlorodihydrofluorescein diacetate (Molecular Probes) for 20 min in the dark. Cells were washed twice with PBS, challenged with heat-killed C. albicans (MOI=10) for 10 or 30 min, and analyzed by FACS.
Prepared cells were blocked with 2.4G2 mAb in staining buffer (PBS containing 0.2% BSA and 0.1% sodium azide) at 4℃ for 20 min, incubated with rat anti-mouse T1/ST2 (clone DJ8)-FITC mAb or rat IgG1κ isotype control at 4℃ for 20 min, and then washed twice with staining buffer. Flow cytometric analysis was performed using a FACS Canto II unit (BD Biosciences). Data were analyzed with FACS Diva (BD Biosciences) and FlowJo software (TreeStar).
To investigate whether IL-33 can affect cytokine production by macrophages in response to C. albicans, peritoneal macrophages were stimulated with heat-killed Candida (MOI=10). We found that C. albicans markedly increased the secretion of IL-6 and TNFα by macrophages at 6 h after challenge and thereafter (Fig. 1). Pre-sensitization of macrophages with IL-33 enhanced the production of these pro-inflammatory cytokines following challenge with C. albicans (Fig. 1).
Since IL-33 signaling is mediated by ST2 (11), we examined whether the expression of ST2 in peritoneal macrophages is regulated by C. albicans. RT-PCR analysis demonstrated that ST2 expression was significantly increased in macrophages 24 h after stimulation with C. albicans (Fig. 2A). We next sought to determine whether the pre-sensitization effect of IL-33 was dependent on ST2. Notably, pre-treatment with a ST2 neutralizing antibody abolished the effect of pre-sensitization with IL-33 on IL-6 and TNFα production following C. albicans infection (Fig. 2B). As ST2 signaling is dependent upon MyD88, we further analyzed the effect of pre-sensitization with IL-33 on pro-inflammatory cytokine production in MyD88 KO peritoneal macrophages. As expected, IL-33 pre-sensitization had no effect on the secreted levels of IL-6 and TNFα following C. albicans challenge in MyD88 KO macrophages (Fig. 2C). These data clearly establish that the effect of pre-sensitization with IL-33 on IL-6 and TNFα production by peritoneal macrophages after C. albicans challenge is mediated through the ST2-MyD88 signaling axis.
We have previously shown that IL-33 pre-sensitization can increase the phagocytic activity of neutrophils against C. albicans (9,10), though little is known about the effect of IL-33 on macrophage phagocytosis. As seen in Fig. 3A, IL-33 priming results in a significant increase in the overall percentage of macrophages containing FITC-conjugated C. albicans at 15 and 30 min post-challenge, yet this was accompanied by only a marginal increase in the FITC intensity observed in C. albicans-engulfed macrophages (Fig. 3B). Notably, IL-33 priming had no effect on the phagocytic activity of MyD88 KO peritoneal macrophages against C. albicans (Fig. 3C). These results indicate that IL-33 priming enhanced macrophage phagocytosis in a MyD88-dependent manner by expanding the population of phagocytic macrophages targeting C. albicans, rather than elevating the phagocytic ability of the individual macrophages.
In addition to phagocytosis, the ability of macrophages to kill engulfed pathogens is also critical for the innate immune response to fungal infection (12,13). Notably, the capacity to generate and utilize intracellular reactive oxygen species (ROS) is crucial for killing C. albicans in phagocytes (14,15). To determine the role of IL-33 priming on ROS production, we analyzed the fluctuations of ROS levels in IL-33-primed peritoneal macrophages in response to fungal challenge. As shown in Fig. 4A, IL-33-primed macrophages displayed higher intracellular ROS levels following challenge when compared to mock-primed controls. Consistent with this result, phagocytosed C. albicans were more rapidly cleared inside IL-33 primed macrophages (Fig. 4B).
We have previously shown that administration of IL-33 prior to peritoneal challenge with a lethal dose of C. albicans prevents sepsis-induced mortality (9,10). In that model, pre-sensitization of the host with IL-33 increases neutrophil responses at multiple stages. Notably, IL-33-primed peritoneal macrophages play a critical role in neutrophil recruitment by producing the CXCR1/2 necessary for neutrophil chemotaxis. The data presented in this study further indicate that peritoneal macrophages may be critical in neutrophil activation, as IL-33-primed macrophages are sufficient to provide the pro-inflammatory cytokines prerequisite for full neutrophil activation. Our results also suggest that IL-33-primed macrophages may contribute to fungal clearance by directly phagocytosing C. albicans, though in vivo experiments are necessary to confirm our in vitro observations.
Although neutrophils play a key role in the anti-fungal defense against C. albicans, macrophages appear to be of equal importance in fungal clearance (16,17). A recent study demonstrates that treatment with IL-13 or PPARγ ligand enhances dectin-1 receptor expression, resulting in induction of phagocytic activity in peritoneal macrophages (18). Interestingly, IL-33 can strengthen M2 macrophage activation to enhance fungal clearance in response to Pneumocystis murina (19). Similarly, our unpublished data revealed that IL-33 pre-treatment can result in a significant increased M2 macrophage polarization in the kidney following C. albicans systemic infection at 3 d post-challenge and thereafter; however, the majority of macrophages present in the kidney at 1 d post-infection display a M1 phenotype. Taken together, this suggests that IL-33 promotes the activation of M1 macrophages during early C. albicans infection, and then slowly converts them toward a M2 macrophage phenotype. Nevertheless, both types of macrophages are known to mediate activities necessary for fungal clearance. This characteristic of IL-33 is unique among cytokines and has a merit in its ability to enhance both resistance and tolerance to C. albicans infection (our unpublished data). In summation, our in vitro data indicate that IL-33 may function as an important mediator of anti-fungal host defenses early after C. albicans infection by promoting fungal clearance.
Figures and Tables
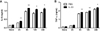 | Figure 1Pre-treatment of peritoneal macrophages with IL-33 increases the production of pro-inflammatory cytokines in response to C. albicans infection. Peritoneal macrophages were pre-treated with 100 ng/ml of IL-33 or PBS for 2 h prior to infection with heat-killed (HK) C. albicans (MOI=10). Levels of IL-6 (A) and TNFα (B) were measured 2 h, 6 h, 12 h, and 24 h after challenge with C. albicans. Data are presented as the mean±SEM of 2-3 trials with similar results. *p<0.05; **p<0.01. |
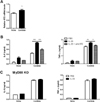 | Figure 2The effect of IL-33 presensitization on pro-inflammatory cytokine production by peritoneal macrophages is dependent on the ST2-MyD88 signaling axis. (A) Peritoneal macrophages were pretreated with 100 ng/ml of IL-33 or PBS for 2 h prior to infection with heat-killed (HK) C. albicans (MOI=10). ST2 mRNA expression was analyzed by performing qRT-PCR 24 h post-challenge. (B) Peritoneal macrophages were pre-treated with 5µg/ml of anti-mouse ST2 mAb or control IgG for 1 h, at which point 100 ng/ml IL-33 was added to the medium. After a 2 h incubation period, macrophages were then challenged with HK-Candida (MOI=10). Levels of IL-6 and TNFα were determined by ELISA with culture supernatants harvested 24 h post-challenge. (C) The same experiments were repeated with peritoneal macrophages isolated from WT and MyD88 KO mice. Data are presented as the mean±SEM of 2-3 independent experiments. *p<0.05; **p<0.01. |
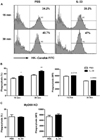 | Figure 3Pre-sensitization of peritoneal macrophages with IL-33 enhances the phagocytic activity of macrophages in a MyD88-dependent manner. (A, B) IL-33-primed peritoneal macrophages were mixed with FITC-labeled, opsonized HK-Candida (MOI=10) for the indicated time periods. Macrophages containing FITC-labeled C. albicans were analyzed by flow cytometry (A). The percentage of FITC-positive macrophages (left column) and the mean fluorescence intensity for FITC of C. albicans-containing macrophages (right column) are quantified in B. (C) The same experiments were repeated with peritoneal macrophages of WT or MyD88 KO mice. Data are presented as the mean±SEM of 2-3 independent experiments. *p<0.05; **p<0.01. |
 | Figure 4IL-33 pre-sensitization enhances ROS production and fungicidal activity in peritoneal macrophages. (A) ROS production was measured as described in Materials and Methods. (B) Peritoneal macrophages were pre-incubated with IL-33 (100 ng/ml) for 3 h before challenge with live, opsonized Candida (MOI=1). Fungal killing activities were determined 15 and 30 min after challenge with C. albicans. Data are presented as the mean±SEM of 2-3 independent experiments. *p<0.05; **p<0.01. |
ACKNOWLEDGEMENTS
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094050 and NRF-2012R1A12008653).
References
1. Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008; 3:e3331.
2. Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010; 10:103–110.

3. Le H, Kim W, Kim J, Cho HR, Kwon B. Interleukin-33: a mediator of inflammation targeting hematopoietic stem and progenitor cells and their progenies. Front Immunol. 2013; 4:104.

5. Lionakis MS, Netea MG. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog. 2013; 9:e1003079.
6. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012; 4:165rv13.

7. Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog. 2012; 8:e1002865.

8. Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Müller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012; 8:e1002811.
9. Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol. 2012; 189:287–295.

10. Kim J, Kim W, Le HT, Moon UJ, Tran VG, Kim HJ, Jung S, Nguyen QT, Kim BS, Jun JB, Cho HR, Kwon B. IL-33-Induced Hematopoietic Stem and Progenitor Cell Mobilization Depends upon CCR2. J Immunol. 2014; doi: 10.4049/jimmunol.1400176.

11. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–490.

12. Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011; 29:1–21.

13. Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012; 80:1304–1313.

14. Missall TA, Lodge JK, McEwen JE. Mechanisms of resistance to oxidative and nitrosative stress: implications for fungal survival in mammalian hosts. Eukaryot Cell. 2004; 3:835–846.

15. Wellington M, Dolan K, Krysan DJ. Live Candida albicans suppresses production of reactive oxygen species in phagocytes. Infect Immun. 2009; 77:405–413.

16. Lewis LE, Bain JM, Lowes C, Gillespie C, Rudkin FM, Gow NA, Erwig LP. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012; 8:e1002578.
17. Marcil A, Harcus D, Thomas DY, Whiteway M. Candida albicans killing by RAW 2647 mouse macrophage cells: effects of Candida genotype, infection ratios, and gamma interferon treatment. Infect Immun. 2002; 70:6319–6329.

18. Galès A, Conduché A, Bernad J, Lefevre L, Olagnier D, Béraud M, Martin-Blondel G, Linas MD, Auwerx J, Coste A, Pipy B. PPARγ controls Dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog. 2010; 6:e1000714.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download