Abstract
Lymphatic vessels are routes for leukocyte migration and fluid drainage. In addition to their passive roles in migration of leukocytes, increasing evidence indicates their active roles in immune regulation. Tissue inflammation rapidly induces lymphatic endothelial cell proliferation and chemokine production, thereby resulting in lymphangiogenesis. Furthermore, lymphatic endothelial cells induce T cell tolerance through various mechanisms. In this review, we focus on the current knowledge on how inflammatory cytokines affect lymphangiogenesis and the roles of lymphatic vessels in modulating immune responses.
Lymphatic vessels (LVs) exist in most vascularized organs (1). LVs contribute to fluid homeostasis by absorbing tissue fluid and draining it into the venous circulation (2,3,4). In addition to fluid homeostasis, LVs are also important for immune surveillance (5). In contrast to blood vessels, lymphatic capillaries are connected by monolayers of lymphatic endothelial cells (LECs) and discontinuous basement membrane; thus, dendritic cells (DCs) can migrate into LVs (6,7). Furthermore, LVs in lymph nodes (LNs) and peripheral tissues are highly plastic; hence, LVs undergo proliferation or remodeling during various pathological conditions such as inflammation (8,9,10,11).
Recent studies have revealed that lymphangiogenesis during inflammation influences the extent of inflammation and immune responses by modulating leukocyte migration and inducing T cell tolerance. In this review, we will describe the inflammatory mediators that affect lymphangiogenesis and the tolerogenic roles of LVs during immune responses.
Several factors regulate lymphangiogenesis (Table I). However, here, we focus only on those factors that have been investigated both in vivo and in vitro. Of them, vascular endothelial growth factors (VEGFs), mainly produced by macrophages are the most well-known and well-studied pro-lymphangiogenic factors. VEGF-C/D and VEGF-A have been reported to induce lymphangiogenesis in various inflammatory models (12,13,14). Blocking the VEGF receptor tyrosine kinase inhibits proliferation and tube formation of umbilical vein endothelial cells and LECs in vitro. Furthermore, in a transgenic mouse model of psoriasis, treatment with a VEGF receptor tyrosine-kinase inhibitor reduces the number of LVs in the skin (15).
In addition to VEGFs, lymphotoxin (LT)-α and LTα1β2 are involved in lymphangiogenesis during infection and lymphoid organ formation. Ectopic expression of LTα promotes the development of LVs within tertiary lymphoid organs. In addition, genetic deletion of LTα1β2 or LTα abrogates development of LVs in the inflamed thyroid (16,17).
Furthermore, interleukin (IL)-8 and hepatocyte growth factor (HGF) promote proliferation and tube formation of LECs without activating VEGF signaling in vitro. IL-8 and HGF promote formation of LVs and improve amelioration of lymphedema in an experimental lymphedema model (18,19).
Fibroblast growth factor (FGF)-2 also exhibits pro-lymphangiogenic activity in the mouse cornea (20). Moreover, FGF-2 induces lymphangiogenesis via both direct and indirect effects. FGF-2 binds LECs and induces proliferation and migration in vitro (21). Furthermore, recent findings suggest that FGF-2 interacts with VEGF-C to induce additional pro-lymphangiogenic activity (22).
Similarly, IL-17 from type 17 T helper (Th17) cells induces lymphangiogenesis in an autoimmune dry eye disease model. IL-17 directly promotes growth of LVs by inducing increased expression of VEGF-D and proliferation of LECs in vitro. Furthermore, in vivo blockade of IL-17 in a model of Th17 dominant autoimmune ocular disease results in a reduction in corneal lymphangiogenesis (23).
By contrast, some inflammatory cytokines have been reported to show anti-lymphangiogenic activity. Interferon (IFN)-γ, which is mainly produced by Th1 cells, inhibits LV formation in an LPS-induced inflammation model. Notably, resolution of increased LVs is dependent on IFN-γ in this model. Furthermore, IFN-γ production by T cells suppresses lymphatic-specific genes in LECs and causes reduction of LV formation in vitro (24).
In addition, in thioglycollate-induced peritonitis and in a lymphedema model, inhibition of transforming growth factor (TGF)-β promotes LV formation (25). Expression of LEC markers, including LYVE-1 and Prox1, is inhibited by TGF-β but is enhanced by a TGF-β type I receptor inhibitor (26).
Therefore, the balance of pro- and anti-lymphangiogenic factors could determine the nature of LVs in various inflammatory conditions (Fig. 1). LVs are involved in immune responses; thus, these results imply that controlling these factors could be a good tool to control lymphangiogenesis and immune responses.
LVs provide pathways for DCs and lymphocyte migration to LNs, thus facilitating inflammation or possible immune tolerance (7,27). For leukocyte trafficking, adhesion molecules like intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 are induced on LECs. Moreover, various chemokines are produced by LECs during inflammation (28,29). Of them, CCL21, which binds to CC-chemokine receptor 7 (CCR7) expressed on DCs and T cells, is the primary determinant of leukocyte migration to LNs (30,31,32). CXCL12 also enhances the migration of CXCR4-expressing leukocytes (33). Furthermore, CX3CL1 was reported to enhance DC transmigration across LECs to LNs (34). Therefore, although CCL21 has a major role in DC migration during inflammation, the expression of other LEC adhesion molecules and chemokines also contributes to migration (29).
In addition to the ability of LECs to control leukocyte trafficking through adhesion molecules, LECs control immune responses via their functions as professional antigen presenting cells (APCs). LECs in LNs constitutively express major histocompatibility complex (MHC)-II molecules. Moreover, they endocytose and cross-present antigens in context with MHC-I molecules to T cells (35,36,37). However, LECs do not express co-stimulatory molecules such as CD80, CD86, OX40L, or 4-1BBL, thus fail to induce T cell activation and proliferation (38,39). Although the lack of co-stimulatory molecules on LECs implies immunological tolerance, further study is needed to elucidate the exact roles of LECs in CD4 T cell activation.
Recently, it was reported that LECs affect not only CD4 T cell tolerance but also CD8 T cell tolerance. LECs express peripheral tissue antigens (PTA) that are restricted to specific tissues such as skin, gut, pancreas, and central nervous system (40,41,42,43,44). Furthermore, LECs induce deletion or abortive proliferation of PTA-specific CD8 T cells through a lack of co-stimulation or engagement with inhibitory molecules such as PD-L1 (38,45,46,47,48). Other co-stimulatory molecules such as HVEM or CD48 are constitutively expressed; thus, their expression suggests that LECs may have immune regulatory roles in steady state as well as in inflammatory conditions (49).
So far, we have discussed inflammation, LV formation, and immune regulation. Different pro- and anti-lymphangiogenic factors are produced by different inflammatory stimuli, and their overall effects determine the extent of lymphangiogenesis. Furthermore, although LECs promote leukocyte migration and enhance immune responses, LECs also attenuate T cell-mediated immune responses by mediating tolerance. LECs attract lymphocytes and DCs through cell adhesion molecules and chemokines. In addition, LECs induce CD8 T cell tolerance through PD-L1 and lack of co-stimulatory molecules. However, mechanisms of CD4 T cell tolerance and roles of other inhibitory molecules on LECs need to be investigated. Finally, controlling lymphangiogenesis could be a novel therapeutic strategy to regulate autoimmunity, enhance tumor immunotherapy, and reduce transplantation rejection.
Figures and Tables
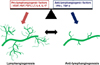 | Figure 1The lymphangiogenic balance between pro-lymphangiogenic factors and anti-lymphangiogenic factors regulates lymphatic vascular homeostasis. Lymphangiogenesis under pathophysiological conditions is associated with increased pro-lymphangiogenic factors and/or decreased anti-lymphangiogenic factors. |
ACKNOWLEDGEMENTS
This study is supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A121145).
References
1. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007; 8:464–478.

3. Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005; 21:457–483.

4. Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006; 126:2167–2177.

5. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008; 6:109–122.

6. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011; 193:607–618.

7. Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009; 206:2925–2935.

8. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005; 438:946–953.

9. He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005; 65:4739–4746.

10. Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006; 119:1755–1760.

11. Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009; 113:5650–5659.

12. Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997; 276:1423–1425.

13. Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001; 20:4762–4773.

14. Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, Nagy JA, Dvorak HF, Yla-Herttuala S, Shibuya M, Alitalo K. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007; 204:1431–1440.

15. Halin C, Fahrngruber H, Meingassner JG, Bold G, Littlewood-Evans A, Stuetz A, Detmar M. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am J Pathol. 2008; 173:265–277.

16. Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007; 104:5026–5031.

17. Mounzer RH, Svendsen OS, Baluk P, Bergman CM, Padera TP, Wiig H, Jain RK, McDonald DM, Ruddle NH. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010; 116:2173–2182.

18. Choi I, Lee YS, Chung HK, Choi D, Ecoiffier T, Lee HN, Kim KE, Lee S, Park EK, Maeng YS, Kim NY, Ladner RD, Petasis NA, Koh CJ, Chen L, Lenz HJ, Hong YK. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis. 2013; 16:29–44.

19. Saito Y, Nakagami H, Morishita R, Takami Y, Kikuchi Y, Hayashi H, Nishikawa T, Tamai K, Azuma N, Sasajima T, Kaneda Y. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006; 114:1177–1184.

20. Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, Chevet E, Bikfalvi A. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013; 121:1229–1237.

21. Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004; 101:11658–11663.

22. Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S, Cao Y. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012; 109:15894–15899.

23. Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, Dana R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011; 118:4630–4634.

24. Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011; 34:96–107.

25. Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010; 177:3202–3214.

26. Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008; 111:4571–4579.
27. Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008; 453:51–55.

28. Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006; 203:2763–2777.

29. Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011; 118:205–215.

30. Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012; 33:271–280.

31. Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009; 69:349–357.

32. MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003; 198:615–621.
33. Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, Yoshiki R, Tamamura H, Fujii N, Inaba K, Tokura Y. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007; 171:1249–1257.

34. Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci. 2013; 126:5259–5270.

35. Amatschek S, Kriehuber E, Bauer W, Reininger B, Meraner P, Wolpl A, Schweifer N, Haslinger C, Stingl G, Maurer D. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 2007; 109:4777–4785.

36. Tripp CH, Haid B, Flacher V, Sixt M, Peter H, Farkas J, Gschwentner R, Sorokin L, Romani N, Stoitzner P. The lymph vessel network in mouse skin visualised with antibodies against the hyaluronan receptor LYVE-1. Immunobiology. 2008; 213:715–728.

37. Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012; 1:191–199.

38. Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, Adler AJ, Chen L, Engelhard VH. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012; 120:4772–4782.

39. Norder M, Gutierrez MG, Zicari S, Cervi E, Caruso A, Guzman CA. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012; 26:2835–2846.

40. Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007; 8:181–190.

41. Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007; 179:993–1003.

42. Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008; 321:843–847.

43. Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010; 207:681–688.

44. Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010; 207:689–697.

45. Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992; 356:607–609.

46. Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001; 194:769–779.
47. Hernandez J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002; 196:323–333.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download