Abstract
Interleukin-18 (IL-18) has been known to induce interferon-γ (IFN-γ) production and promote Th1 immunity. Although mammalian IL-18 has been characterized in great detail, the properties and application of chicken IL-18 remain largely uninvestigated as of yet. In this study, we evaluated the immunomodulatory properties of Salmonella enterica serovar Typhimurium expressing chicken interleukin-18 (chIL-18) on immune responses induced by avian influenza (AI) and Newcastle disease (ND) vaccines. After oral administration of S. enterica serovar Typhimurium expressing chIL-18, chickens were vaccinated intramuscularly with the recommended dose of either inactivated AI H9N2 vaccine or ND (B1 strain) vaccine. Chickens receiving a primary vaccination were boosted using the same protocol 7 days later. Humoral and cell-mediated immune responses were evaluated in terms of HI antibody titers and proliferation and mRNA expression of IFN-γ and IL-4 of peripheral blood mononuclear cells (PBMC) in response to specific antigen stimulation. According to our results, oral administration of S. enterica serovar Typhimurium expressing chIL-18 induced enhanced humoral and Th1-biased cell-mediated immunity against AI and ND vaccines, compared to that of chickens received S. enterica serovar Typhimurium harboring empty vector. Therefore, we conclude that our proposed vaccination regimen using inactivated AI and ND viruses along with oral administration of S. enterica serovar Typhimurium expressing chIL-18 may provide a novel approach in protecting chicken from currently circulating AI and ND virus strains.
Avian influenza virus (AIV) is an enveloped virus that belongs to the Orthomyxoviridae family and has an eight segmented, single stranded, negative sense RNA genome. Among the proteins encoded by the genome, there are two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). AIV is classified into subtypes according to the combination of 16 HA and 9 NA molecules (1). The H9N2 subtype low pathogenic avian influenza (LPAI) is one of the most prevalent avian diseases worldwide, and was first documented in 1996 in Korea. This disease caused serious economic loss in Korea's poultry industry. This particular subtype has attracted great concern due to its wide host range (2), chance of genetic reassortment (3) and possible avian-to-human transmission (4) rather than industrial losses. Newcastle disease (ND) is another serious avian disease that particularly affects chickens worldwide and causes severe economic losses in the poultry industry. The causative agent of the disease, Newcastle disease virus (NDV) also known as avian paramyxovirus type 1, is a member of the Paramyxoviridae and contains a non-segmented single stranded RNA genome of negative polarity (5).
In addition to good biosecurity practices, control of LPAI and ND primarily consists in preventive vaccination of flocks and culling of infected and at risk of being infected birds (protection zone). Current vaccination programs include the use of attenuated (live) vaccines followed by inactivated (killed) vaccines, in order to induce a good protective immunity while producing minimal adverse effects in birds. Both vaccines have their advantages and disadvantages, which have been reviewed previously (6). Nevertheless, the current vaccines and vaccination strategies protect against morbidity and mortality and significantly reduce but do not stop the infection and the viral excretion, which is critical for controlling the spread of the disease (7). Another limitation to their efficacy is that the current vaccines cannot provide better protection against more recent circulating viruses (7,8). More importantly, in the case of use of live virus vaccine, it is not possible to distinguish vaccine viruses from field isolates which makes sero-surveillance more complicated. Therefore, a marker vaccine or DIVA (Differentiating Infected from Vaccinated animals) vaccine has been introduced as different approach to overcome such limitation (9). However, these may interfere with maternally derived antibody (MDA) and therefore, marker vaccine requires serological screening of MDA to determine appropriate vaccination time which makes it complicated to use in the field. Therefore, an improved vaccination strategy is urgently required for conventional chickens provided with MDA to overcome the limitations of current vaccination.
Cytokines are natural mediators of innate and adaptive immune responses which play a crucial role in controlling the immune system. The use of chicken cytokines is becoming more feasible with the recent cloning of a number of cytokine genes (10). Interleukin-18 (IL-18), originally known as interferon-γ (IFN-γ)-inducing factor, provides an important link between the innate and adaptive immune responses through promoting IFN-γ production and thereby inducing Th1 immune responses (11). Recently, we also reported that chIL-18 combined with chIFN-α had enhanced antiviral and immunomodulatory properties against AIV H9N2 in vivo when administered orally using attenuated S. enterica serovar Typhimurium strain χ8501 (12,13). Recombinant chicken IL-18 (chIL-18) protein also has the ability to act as a potent adjuvant when co-administered with cell-cultured Newcastle disease vaccine (14). Recently, it is reported that chicken IL-15 and IL-18 used as genetic adjuvants improve the immune responses induced from the AIV H5 DNA vaccination in chickens as evaluated by antibody production, T cell responses and cytokine production (15). However, the properties and practical application of chIL-18 remain largely uninvestigated as of yet. Furthermore, the mass administration of chIL-18 to industrial animals such as poultry is limited by cost, labor, and time, as well as protein stability. Therefore, it is necessary to develop an effective delivery system for the mass administration of chIL-18 to overcome these limitations. To this end, we investigated the immunomodulatory properties of chIL-18 as delivery carrier using live attenuated Salmonella enterica serovar Typhimurium in chickens immunized with inactivated avian influenza H9N2 and Newcastle disease B1strain vaccines.
SPF White Leghorn layer chickens were obtained from OrientBio (Seongnam-Si, Korea), and reared with formulated commercial feed and water provided ad libitum throughout the experimental period. All experimental and animal management procedures were undertaken in accordance with the requirements of the Animal Care and Ethics Committees of Chonbuk National University. The animal facility of Chonbuk National University is fully accredited by the National Association of Laboratory Animal Care.
LPAIV H9N2 strain, A/Chicken/Korea/01310/2001(01310), and Newcastle disease virus B1 strain were provided by the National Veterinary Research and Quarantine Service of the Republic of Korea and used for the challenge experiments. Both the viruses were propagated by inoculating them into the allantoic cavity of 10-day-old embryonated eggs. Allantoic fluid was harvested 96 h after inoculation in each case and the infectious viral titers were determined separately using 10-day-old embryonated eggs.
Attenuated S. enterica serovar Typhimurium expressing chIL-18 (χ8501/chIL-18) was constructed by cloning chIL-18 gene with reverse transcriptase-PCR, as described elsewhere (12). Attenuated S. enterica serovar Typhimurium χ8501 (hisG Δcrp-28 ΔasdA16) was used as the host bacteria to deliver chIL-18 protein using pYA3560 and pYA3493 vectors. S. enterica serovar Typhimurium cultures were grown at 37℃ in Lennox broth, Luria-Bertani (LB) broth, or on LB agar. The expression of chIL-18 by S. enterica serovar Typhimurium was identified by immunoblotting following gel separation of prepared protein by SDS-PAGE (Data not shown). Furthermore, the biological activity of secreted chIL-18 protein from χ8501/chIL-18 in culture supernatant was evaluated by Griess assay (16), where secreted chIL-18 was shown to induce nitric oxide (NO) production by HD-11 cells (data not shown).
A total of 20 SPF chickens (22-days-old) were divided randomly into three groups. The first group (n=5) was a negative control orally administered vehicle (PBS containing 0.01% gelatin) without S. enterica serovar Typhimurium expressing chIL-18. The second group (n=5) was orally administered S. enterica serovar Typhimurium harboring pYA3560 vector (χ8501/pYA3560, 109 cfu/chicken) as a control for the empty pYA3560 vector. The remaining two groups (n=5) were orally administered two different doses of S. enterica serovar Typhimurium expressing chIL-18 (χ8501/chIL-18, 109 and 1011 cfu/chicken). Three days after treatment, the 25-day-old chickens from all groups except the negative control, were vaccinated intramuscularly with the recommended dose of AIV H9N2 inactivated vaccine (PoulShot® Flu H9N2; Choong Ang Vaccine Inc., Daejeon, Korea). With regard to ND vaccination, NDV was inactivated with 0.1% formaline and used for intramuscular immunization. Chickens receiving a primary vaccination were boosted using the same protocol 7 days later. Blood samples were collected 7 days after the primary vaccination and 7 and 14 days after booster vaccinations followed by sera separation. Peripheral blood mononuclear cells (PBMCs) were enriched from the blood of vaccinated chickens using OptiPrep™ (13.8% iodixanol) 14 days post-booster vaccination, according to the manufacturer's instructions (Axis-Shield, Oslo, Norway). Another experiment was performed using the same experimental design to evaluate the immunomodulatory functions of recombinant chIL-18 in ND vaccination.
To determine HI titers of the sera samples collected from vaccinated chickens, HI tests were performed separately with AIV H9N2 (01310) and NDV B1 antigens using a standard method. The geometric means of serum HI titers obtained from each group were defined as the reciprocal logarithm in a base of 2 of the highest serum dilution completely inhibiting agglutination.
Antigen-specific proliferation of PBMCs was assessed by measuring viable cell ATP bioluminescence in both the experiments. Briefly, PBMCs (responder) were prepared from chickens vaccinated with either inactivated AIV H9N2 vaccine or formalin-inactivated NDV B1 strain vaccine, as previously described (17). Responder cells were cultured together with stimulator cells at three different ratios. Autologous PBMCs (106 cells/ml), which were isolated from corresponding chickens before vaccination and kept at liquid nitrogen tank, had been pulsed with either ultraviolet (UV)-inactivated AIV H9N2 antigen (2.5×102 HA units/ml) or UV-inactivated NDV B1 antigen (2.5×102 HA units/ml) for 3 h and followed by treatment with mitomycin C (25 µg/ml) for 5 min, and were employed as stimulator cells. Following 72 h incubation, the proliferated cells were evaluated using a ViaLight® Cell proliferation assay kit (Cambrex Bio Science, Rockland, ME, USA), according to the manufacturer's instructions. PBMC stimulators that were not pulsed with either UV-inactivated AIV H9N2 antigen or UV-inactivated NDV B1 antigen were used for negative control.
Real-time quantitative RT-PCR (qRT-PCR) analysis was used for determining the mRNA expression levels of chicken IFN-γ (chIFN-γ) and IL-4 (chIL-4) from PBMC in response to specific antigen stimulation. Briefly, total RNA were extracted from collected samples using total RNA extraction kits (iNtRON), according to the manufacturer's instructions, and then subjected to real-time qRT-PCR using a One-Step SYBR® qRT-PCR reagent kit (Takara, Shiga, Japan) and primers specific for the IFN-γ, IL-4 (17). RT and real-time PCR amplification of targeted genes were carried out under the same reaction conditions and temperature cycles, as described previously (17). After the reaction cycle was completed the temperature was increased from 50℃ to 95℃ at a rate of 0.2℃/15 s and fluorescence was measured every 5 s to construct a melting curve that was used to confirm the authenticity of the amplified products. A control sample that contained no template RNA was run with each assay, and qRT-PCR data was normalized using the commonly used reference gene, GAPDH (17). All data were analyzed using CFX96™ manager software version 1.6 (Bio-Rad).
All data were expressed as the average±standard error. Differences between the two group were compared using an unpaired two-tailed Student's t-test. Differences between multiple groups were compared using two-way analysis of variance (ANOVA) using SAS version 9.1 (SAS Institute Inc., Cary, NS, USA). A p-value<0.05 was considered to indicate a statistically significant difference between the groups.
In order to examine the humoral immune responses in AIV H9N2-vaccinated chickens with or without oral administration of S. enterica serovar Typhimurium expressing chIL-18, groups of chickens (n=5) treated with χ8501/chIL-18 at two different doses (109 and 1011 cfu/chicken) were vaccinated twice with AIV H9N2 inactivated vaccine. Sera samples were collected 7 days after primary vaccination and 7 and 14 days after booster vaccination and used for the determination of HI antibody titers (Fig. 1A). Significantly enhanced HI antibody levels were observed at all three time points in the sera of χ8501/chIL-18-administered chickens at both doses, compared to that of χ8501 (pYA3560)-treated chickens (Fig. 1B). Significant difference in HI antibody levels was also existed between two replications (109 and 1011 cfu). To check whether χ8501/chIL-18 has similar immunostimulatory effects in other vaccine viruses, we performed another similar experiment using formalin-inactivated NDV (B1 strain) vaccine and found that significantly enhanced HI antibody levels were also observed in the sera of chickens that received χ8501/chIL-18 (109 and 1011 cfu) orally, compared to that of χ8501 (pYA3560)-treated chickens (Fig. 2). It is noted in this experiment that no significant difference in HI antibody levels was observed between two replications (109 and 1011 cfu per chicken), which indicated that χ8501/chIL-18 was effective at both the doses (109 and 1011 cfu). Therefore, these results indicate that oral administration of S. enterica serovar Typhimurium expressing chIL-18 produced enhanced humoral immune responses against AI and ND vaccines.
To evaluate the modulation of cellular immune responses by S. enterica serovar Typhimurium expressing chIL-18, PBMCs were prepared from AIV H9N2-vaccinated chickens that received oral administration of χ8501/chIL-18 and subsequently subjected to stimulation with autologous PBMCs that had been previously pulsed with UV-inactivated AIV H9N2 antigen. PBMCs of chickens that received χ8501/chIL-18 (109 and 1011 cfu per chicken) orally prior to AI vaccination were found to have significantly enhanced proliferation upon AIV H9N2 antigen-specific stimulation, compared to PBMCs from chickens that received χ8501/pYA3560 (vehicle) and vaccine (Fig. 3A). However, no significant difference existed between two replications (109 and 1011 cfu). Additionally, the mRNA expression levels of IFN-γ and IL-4 in PBMCs were determined by real-time qRT-PCR following stimulation with AIV H9N2 antigen. Both IFN-γ and IL-4 mRNA levels in PBMCs prepared from chickens that received χ8501/chIL-18 (109 and 1011 cfu) orally were significantly enhanced, compared to chickens that received S. enterica serovar Typhimurium harboring empty pYA3560 vector plus AI vaccine (Fig. 3B). More importantly, the expression of IFN-γ mRNA was more significantly up-regulated than IL-4 mRNA with administration of χ8501/chIL-18 at both the doses (109 and 1011 cfu), which indicated Th1-biasness.
We further evaluated the immune modulatory properties of recombinant chIL-18 in another important vaccine virus namely NDV (B1 strain). Our results revealed that significantly enhanced proliferation of PBMCs, prepared from chickens that received χ8501/chIL-18 (109 and 1011 cfu per chicken) orally prior to ND vaccination, was observed following UV-inactivated autologous antigen stimulation, compared to PBMC prepared from chickens that received S. enterica serovar Typhimurium harboring empty pYA3560 vector plus ND vaccine (Fig. 4A). The mRNA expression levels of IFN-γ and IL-4 in PBMCs were also determined by real-time qRT-PCR following stimulation with NDV (B1) antigen. Similar to AI vaccination experiment, both IFN-γ and IL-4 mRNA levels in PBMCs prepared from chickens that received χ8501/chIL-18 (109 and 1011 cfu) orally were significantly enhanced, compared to chickens that received S. enterica serovar Typhimurium harboring empty pYA3560 vector plus ND vaccine (Fig. 4B) and the expression of IFN-γ mRNA was more significantly up-regulated than IL-4 mRNA with administration of χ8501/chIL-18 at both the doses (109 and 1011 cfu). Taken together, our results indicate that oral administration of S. enterica serovar Typhimurium expressing chIL-18 induces enhanced Th1-biased immunity against AI & ND vaccines, compared to chickens that receive S. enterica serovar Typhimurium harboring empty pYA3560 vector plus AI or ND vaccine.
The continuous outbreaks of fatal AI and ND in commercial poultry flocks in many parts of the world indicate that routine vaccination in the field often fails to induce sufficiently high levels of immunity to control those diseases. Especially, in region where AI and ND are enzootic and where there is high pressure from the field, the need for very early immunization is hampered by the interference of live vaccine viruses with a usually high level of MDA. In such cases, early stimulation of immune system using immunomodulatory cytokines followed by immunization with an inactivated vaccine might be a novel approach. Therefore, in the present study, we orally administered Salmonella enterica serovar Typhimurium expressing chicken interleukin-18 in chickens three days prior to each immunization with either inactivated AI H9N2 or inactivated ND (B1strain) vaccines and evaluated the immune modulation induced by recombinant chIL-18. According to our results, oral administration of Salmonella enterica serovar Typhimurium expressing chicken interleukin-18 modulated both humoral and Th1-biased cell-mediated immunity against avian influenza and Newcastle disease vaccines.
Interleukin-18 (IL-18), originally known as interferon-γ (IFN-γ)-inducing factor, shares properties with IL-12 and both cytokines act synergistically to promote IFN-γ production, which plays an important role in inducing Th1 immune responses (11). When co-administered with Al(OH)3-adsorbed IL-12, IL-18 augmented antigen-specific Th1 type responses (18). Pollock et al. (18) previously demonstrated in mice that IL-18 facilitates Al(OH)3-induced IL-4 production. Bacterially expressed chIL-18 is capable of inducing both the synthesis of chicken IFN-γ in cultured primary chicken spleen cells and the proliferation of CD4+ T cells (19,20). In addition, the purified Escherichia coli-expressed recombinant chIL-18 significantly enhanced antibody responses to Clostridium perfringens α-toxoid and Newcastle disease (ND) virus antigens (21). According to the above mentioned findings it is conceivable that oral administration of Salmonella enterica serovar Typhimurium expressing chIL-18 might modulate both humoral and Th1-biased cell-mediated immunity against avian influenza and Newcastle disease vaccines. Our present findings are also supported by our recently published data that chIL-18 combined with chIFN-α had enhanced antiviral and immunomodulatory properties against AIV H9N2 in vivo when administered orally using attenuated S. enterica serovar Typhimurium strain χ8501 (13). Furthermore, recently it is reported that recombinant chIL-18 potentiated both the humoral and cell mediated immune responses against recombinant Newcastle disease virus vaccine (21,22) which directly supports our findings. The use of attenuated S. enterica serovar Typhimurium strain χ8501 as a successful mammalian and avian cytokine genes delivery system is well addressed at our laboratory in our several recent publications (13,17,23,24). According to our published data and other related published data, it is believed that the Salmonella bacteria used for cytokine delivery can persist in chickens for 3~7 weeks, depending on age of chickens, and can provide continuous long term protection against virus infection (12,25,26). Therefore, our present findings provide a significant in site into the use of recombinant chIL-18 in case of vaccinations using inactivated AI and ND viruses and thereby invent a novel approach in vaccination regimen using inactivated or killed vaccine. Although we did not study the protective efficacy of such vaccinations in our present study due to some limitation, we studied the protective efficacy of enhanced immune response against inactivated AIV H9N2 vaccine modulated by oral co-administration of chIL-18 and chIFN-α using S. enterica serovar Typhimurium strain χ8501 and found 100 percent protection of SPF chicken following lethal challenge with high dose of AIV H9N2 (01310) (1010.83 egg infective dose [EID]50/chicken).
Our previous report (13,17) and present study demonstrated the values of attenuated Salmonella vaccine in the oral delivery of immunomodulatory cytokines. Genetically modified Lactococcus lactis secreting bioactive cytokine has been found to be a useful tool for live mucosal delivery (27,28). L. lactis is a safe vector for delivery of foreign genes via foodstuffs to digestive tract without colonization (27,28), yet effectively induces local immune responses. In contrast, live Salmonella vaccine can colonize gut-associated lymphoid tissue and visceral nonlymphoid and lymphoid tissues following oral administration, and subsequently stimulate local and systemic immune responses. Therefore, attenuated live Salmonella vaccine may be a useful means to deliver bioactive cytokines to systemic as well as mucosal lymphoid tissues. In conclusion, we believe that our proposed novel vaccination regimen using inactivated AI and ND viruses along with oral administration of S. enterica serovar Typhimurium expressing chIL-18 will provide protection of chicken from currently circulating AI and ND virus strains. However, further studies on protective function against these diseases especially ND is required to provide a final recommendation.
Figures and Tables
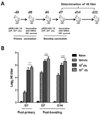 | Figure 1The serum hemagglutination inhibition (HI) antibody titers in chickens vaccinated intramuscularly with inactivated AIV H9N2 vaccine with or without oral administration of S. entericaserovar Typhimurium expressing chIL-18. (A) Diagram for vaccination schedule using S. entericaserovar Typhimurium expressing chIL-18. Groups of chickens were administered S. enterica serovar Typhimurium expressing chIL-18 (109 and 1011 cfu/chicken) and vaccinated with inactivated AIV H9N2 vaccine three days later. The vaccinations were performed by same protocol twice at 7-day intervals. (B) HI antibody titers in sera of vaccinated chickens. Serum samples collected from chickens of all groups 7 days after primary vaccination and 7 and 14 days after booster vaccination were subjected to HI testing. Data are expressed as reciprocal log2 of the geometric average and SEM of HI titers obtained from five chickens per group. ***p<0.001 compared to vehicle group treated with control bacteria. |
 | Figure 2The serum hemagglutination inhibition (HI) antibody titers in chickens vaccinated intramuscularly with inactivated NDV (B1 strain) vaccine with or without oral administration of S. entericaserovar Typhimurium expressing chIL18. Groups of chickens were administered S. entericaserovar Typhimurium expressing chIL-18 (109 and 1011 cfu/chicken) and vaccinated with inactivated NDV (B1 strain) vaccine three days later. The vaccinations were performed by same protocol twice at 7-day intervals. Serum samples collected from chickens of all groups 7 days after primary vaccination and 7 and 14 days after booster vaccination were subjected to HI testing. Data are expressed as reciprocal log2 of the geometric average and SEM of HI titers obtained from six chickens per group. ***p<0.001 compared to vehicle group treated with control bacteria. |
 | Figure 3Enhanced Th1-biased immunity in chickens vaccinated with inactivated AIV H9N2 vaccine following oral administration of S. entericaserovar Typhimurium expressing chIL-18. (A) AIV H9N2 antigen-specific proliferation of PBMCs. Groups of chickens were administered S. enterica serovar Typhimurium expressing chIL-18 (109 and 1011 cfu/chicken) and vaccinated with inactivated AIV H9N2 vaccine three days later. The vaccination was performed by same protocol twice at 7-day intervals. PBMCs (responders) were prepared from chickens 14 days after booster vaccination, and subsequently stimulated with autologous naïve PBMCs (stimulators) that had been pulsed with uv-inactivated AIV H9N2 antigen. Antigen-specific proliferation of PBMCs was assessed by measuring viable cell ATP bioluminescence following incubation for 72 h. (B) The expression of IFN-γ and IL-4 mRNA by PBMCs following stimulation with AIV H9N2 specific antigen. Total RNA was extracted from PBMCs stimulated with specific antigen for 72 h, and subjected to real-time qRT-PCR to determine the expression of IFN-γ and IL-4. Data show the average and SEM of IFN-γ and IL-4 expression obtained from five chickens per group, after normalized to GAPDH. ***p<0.001 compared to vehicle group treated with control bacteria. |
 | Figure 4Enhanced Th1-biased immunity in chickens vaccinated with inactivated NDV (B1 strain) vaccine following oral administration of S. entericaserovar Typhimurium expressing chIL-18. (A) NDV (B1 strain) antigen-specific proliferation of PBMCs. Groups of chickens were administered S. entericaserovar Typhimurium expressing chIL-18 (109 and 1011 cfu/chicken) and vaccinated with inactivated NDV (B1 strain) vaccine three days later. The vaccination was performed by same protocol twice at 7-day intervals. PBMCs (responders) were prepared from chickens 14 days after booster vaccination, and subsequently stimulated with autologous naïve PBMCs (stimulators) that had been pulsed with uv-inactivated NDV (B1 strain) antigen. Antigen-specific proliferation of PBMCs was assessed by measuring viable cell ATP bioluminescence following incubation for 72 h. (B) The expression of IFN-γ and IL-4 mRNA by PBMCs following stimulation with NDV (B1 strain) specific antigen. Total RNA was extracted from PBMCs stimulated with specific antigen for 72 h, and subjected to real-time qRT-PCR to determine the expression of IFN-γ and IL-4. Data show the average and SEM of IFN-γ and IL-4 expression obtained from six chickens per group, after normalized to GAPDH. ***p<0.001 compared to vehicle group treated with control bacteria. |
ACKNOWLEDGEMENTS
This study was supported by the Mid-career Research Program (2012R1A2A1A036702) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology.
References
1. Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005. 79:2814–2822.

2. Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000. 74:3–13.

3. Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, Lim W, Webster RG, Yuen KY, Peiris JS, Guan Y. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005. 43:5760–5767.

4. Li KS, Xu KM, Peiris JS, Poon LL, Yu KZ, Yuen KY, Shortridge KF, Webster RG, Guan Y. Characterization of H9 subtype influenza viruses from the ducks of southern China: a candidate for the next influenza pandemic in humans? J Virol. 2003. 77:6988–6994.

5. Alexander DJ. Saif YM, editor. Newcastle disease, other avian Paramyxoviruses, and Pneumovirusinfections. Diseases of poultry. 2003. 11th ed. IA: IowaState Press;63–87.
6. Senne DA, King DJ, Kapczynski DR. Control of Newcastle disease by vaccination. Dev Biol (Basel). 2004. 119:165–170.
7. van Boven M, Bouma A, Fabri TH, Katsma E, Hartog L, Koch G. Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Pathol. 2008. 37:1–5.
8. Kapczynski DR, King DJ. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine. 2005. 23:3424–3433.

10. Kaiser P. Advances in avian immunology--prospects for disease control: a review. Avian Pathol. 2010. 39:309–324.

12. Rahman MM, Uyangaa E, Han YW, Kim SB, Kim JH, Choi JY, Eo SK. Enhancement of Th1-biased protective immunity against avian influenza H9N2 virus via oral co-administration of attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α and interleukin-18 along with an inactivated vaccine. BMC Vet Res. 2012. 8:105.

13. Rahman MM, Uyangaa E, Han YW, Kim SB, Kim JH, Choi JY, Eo SK. Oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α and interleukin-18 enhances the alleviation of clinical signs caused by respiratory infection with avian influenza virus H9N2. Vet Microbiol. 2012. 157:448–455.

14. Hung LH, Li HP, Lien YY, Wu ML, Chaung HC. Adjuvant effects of chicken interleukin-18 in avian Newcastle disease vaccine. Vaccine. 2010. 28:1148–1155.

15. Lim KL, Jazayeri SD, Yeap SK, Alitheen NB, Bejo MH, Ideris A, Omar AR. Co-administration of avian influenza virus H5 plasmid DNA with chicken IL-15 and IL-18 enhanced chickens immune responses. BMC Vet Res. 2012. 8:132.

16. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988. 141:2407–2412.
17. Rahman MM, Uyangaa E, Han YW, Kim SB, Kim JH, Choi JY, Yoo DJ, Hong JT, Han SB, Kim B, Kim K, Eo SK. Oral administration of live attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α alleviates clinical signs caused by respiratory infection with avian influenza virus H9N2. Vet Microbiol. 2011. 154:140–151.

18. Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology. 2003. 108:137–143.

19. Schneider K, Puehler F, Baeuerle D, Elvers S, Staeheli P, Kaspers B, Weining KC. cDNA cloning of biologically active chicken interleukin-18. J Interferon Cytokine Res. 2000. 20:879–883.

20. Göbel TW, Schneider K, Schaerer B, Mejri I, Puehler F, Weigend S, Staeheli P, Kaspers B. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in anonmammalian species. J Immunol. 2003. 171:1809–1815.

21. Degen WG, van Zuilekom HI, Scholtes NC, van Daal N, Schijns VE. Potentiation of humoral immune responses to vaccine antigens by recombinant chicken IL-18 (rChIL-18). Vaccine. 2005. 23:4212–4218.

22. Su BS, Shen PC, Hung LH, Huang JP, Yin HS, Lee LH. Potentiation of cell-mediated immune responses against recombinant HN protein of Newcastle disease virus by recombinant chicken IL-18. Vet Immunol Immunopathol. 2011. 141:283–292.

23. Kim SJ, Kim SB, Han YW, Uyangaa E, Kim JH, Choi JY, Kim K, Eo SK. Co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interleukin-18 and interferon-α provides enhanced Th1-biased protective immunity against inactivated vaccine of pseudorabies virus. Microbiol Immunol. 2012. 56:529–540.

24. Lee BM, Han YW, Kim SB, Rahman MM, Uyangaa E, Kim JH, Roh YS, Kim B, Han SB, Hong JT, Kim K, Eo SK. Enhanced protection against infection with transmissible gastroenteritis virus in piglets by oral co-administration of live attenuated Salmonella enterica serovar Typhimurium expressing swine interferon-α and interleukin-18. Comp Immunol Microbiol Infect Dis. 2011. 34:369–380.

25. Beal RK, Powers C, Davison TF, Barrow PA, Smith AL. Clearance of enteric Salmonella enterica serovar Typhimurium in chickens is independent of B-cell function. Infect Immun. 2006. 74:1442–1444.

26. Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol. 2004. 100:151–164.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download