Abstract
Previously we showed that biodegradable nanoparticles containing poly-IC or CpG oligodeoxynucleotide (ODN) together with ovalbumin (OVA) were efficient at inducing MHC-restricted presentation of OVA peptides in dendritic cells. The CTL-inducing activities of the nanoparticles were examined in the present study. Nanoparticles containing poly-IC or CpG ODN together with OVA were prepared using biodegradable polymer poly(D,L-lactic acid-co-glycolic acid), and then were opsonized with mouse IgG. The nanoparticles were injected into the tail vein of mice, and 7 days later the OVA-specific CTL activities were measured using an in vivo CTL assay. Immunization of mice with the nanoparticles containing poly-IC or CpG ODN together with OVA elicited potent OVA-specific CTL activity compared to those containing OVA only. In accordance with these results, nanoparticles containing poly-IC or CpG ODN together with OVA exerted potent antitumor activity in mice that were subcutaneously implanted with EG7.OVA tumor cells. These results show that encapsulation of poly-IC or CpG ODN together with antigen in biodegradable nanoparticles is an effective approach for the induction of potent antigen-specific CTL responses in vivo.
Induction of robust cytotoxic T lymphocyte (CTL) responses is essential for the immunotherapy against cancers or viral infections. Naïve CD8 T cells become activated when their receptors recognize antigens presented by professional antigen presenting cells in the context of MHC-I molecules (1). The cross-presentation pathway, which allows MHC-I-restricted presentation of exogenous antigen, appears to be an obligatory mechanism for the generation of CTL responses to antigens that are expressed only in nonprofessional antigen presenting cells (APCs) (2-6). In the absence of such a mechanism, viral or tumor antigens expressed in nonprofessional APCs could escape immunosurveilance because CTL responses can only be induced efficiently for the antigens presented via class I MHC molecules on professional APCs (2-6).
Delivery of antigens using nanoparticles prepared from biodegradable polymers such as poly(D, L-lactic acid-co-glycolic acid) (PLGA) into professional APCs is an efficient method for the induction of potent CTL responses. We and others have also shown that PLGA particle-mediated antigen delivery enhances and prolongs the MHC class I-restricted presentation of the exogenous antigens (cross-presentation) in dendritic cells (DCs) (7-10). PLGA-nanoparticles have also been shown to deliver antigens to APCs efficiently and generate Th1-type immune responses even against poor immunogens (11,12). In our hand, antigens encapsulated with PLGA were at least 100 times more effective in inducing MHC-I-restricted presentation of exogenous antigen in DCs (13). Another advantage of nanoencapsulation would also be the protection of the encapsulated antigens and the immunomodulators from degradation by serum enzymes (14).
Recently, we showed that nanoparticles containing poly-IC or CpG ODN together with ovalbumin (OVA) increases and prolongs both MHC class I- and class II-restricted presentation of OVA peptides in DCs (9). In the present study, we examined the capability of the nanoparticles to induced OVA-specific CTL responses in mice.
Nanoparticles containing poly-IC or CpG ODN together with OVA were prepared using a biocompatible/biodegradable polymer, PLGA, as described earlier (9). The poly-IC used in the present study was purchased from Invivogen (San Diego, CA, USA). Unmethylated CpG oligodeoxynucleotide (ODN), 5'-TCC ATG ACG TTC CTG ATG CT-3', was synthesized by the Bionics Co. Ltd (Seoul, Korea). The amounts of poly-IC and CpG DNA contained in the nanoparticles were 1.40 and 2.01µg/mg nanoparticles, respectively. The average content of OVA was 21.68µg/mg nanoparticles. For opsonization, OVA-specific mouse IgG (mIgG) or was attached covalently to the nanoparticles using (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide) (EDC, Pierce, Rockford, IL, USA) as previously described (9).
The CTL inducing activities of the nanoparticles containing OVA only (NP[OVA]), both OVA and poly-IC (NP[OVA+I:C], or both OVA and CpG ODN (NP[OVA+CpG]) were compared in mice. In this experiment, the nanoparticles were injected intravenously into tail veins of mice (100µg as OVA/mouse). Seven days later, an in vivo CTL assay was performed in the mice using CFSE-labeled syngeneic target cells, as described in detail in the earlier paper (9). Fig. 1A shows representative histograms of the cells isolated from the spleens. Addition of poly-IC or CpG to OVA-nanoparticles significantly increased their ability to induce OVA-specific CTLs in the spleens (Fig. 1B) and lymph nodes (Fig. 1C). Immunization of mice with both NP[OVA+I:C] and NP[OVA+CpG] further increased OVA-specific CTLs in the spleens and lymph nodes.
To confirm that the induction of OVA-specific CTL activity is sufficient to engender antitumor activity, mice were immunized with the nanoparticles containing bovine serum albumin (BSA) only (NP[BSA]), OVA only (NP[OVA]), both OVA and poly-IC (NP[OVA+I:C], or both OVA and CpG ODN (NP[OVA+CpG]), intravenously into tail veins of the mice (10 µg as OVA or BSA/mouse). Seven days later, the mice were subcutaneously implanted with EG7.OVA tumor cells (5×105/mouse), which is a mouse lymphoma expressing OVA. Two days later, the mice were immunized with the same nanoparticles intravenously into tail veins of the mice. The tumor size was measured with a slide caliper and expressed as a tumor index, determined as the square root of (major axis×minor axis). As shown in Fig. 2A, the growth of tumors was obvious from day 14 after tumor cell implantation, and reached to average size of 3.76 cm3 at day 25 in the mice that were immunized with the nanoparticles containing an irrelevant protein, BSA. Immunization of the mice with NP[OVA] significantly reduced the size of the tumors. The average size of the tumors was 2.92 cm3 at day 25 in the mice that were immunized with the NP[OVA]. Addition of poly-IC or CpG to OVA-nanoparticles significantly reduced the size of the tumors. Immunization of mice with both NP[OVA+I:C] and NP[OVA+CpG] almost completely reduced the development of the tumors.
The antitumor efficacy of the combined use of NP[OVA+I:C] and NP[OVA+CpG] was further confirmed in mice implanted with EG7.OVA tumor cells. In this experiment, mice were subcutaneously implanted with the tumor cells, and then both types of the nanoparticles were mixed in a 1:1 ratio, and injected into the tumor mass on 10, 12 and 14 days after the tumor implantation (20µg as OVA/mouse). As shown in Fig. 2B. intratumoral injection of both NP[OVA+I:C] and NP[OVA+CpG] completely inhibited the formation of tumor mass.
Because the EG7.OVA cells express only MHC-I molecules and not MHC-II molecules, it is reasonable to speculate that the antitumor activity shown by the nanoparticles is the reflection of the OVA-specific CTL activity (15). In addition, it is noteworthy to note that the TLR agonists, poly-IC and CpG, were entrapped inside the PLGA-nanoparticles. Encapsulation prevents not only the systemic effects of the TLR agonists, but also the enzymatic degradation of the TLR agonists (9,14-18).
Robust induction of CTL activity is important in the immunotherapy of tumors and viral infections. Recently, our laboratory has been involved in the development of strategies to enhance the MHC-I-restricted antigen presentation of exogenous antigen (8,9,19,20). We showed that nanoencapsulation of poly-IC or CpG together with OVA is an efficient approach to increase and prolong the MHC-restricted presentation of OVA peptides in dendritic cells (9). We also showed that IgG-opsonized PLGA-nanoparticles with a mean size of 1.1µm would be the choice of biodegradable carriers for the targeted-delivery of protein antigens for cross-priming in vivo (20). The present study confirms that nanoparticles containing poly-IC or CpG ODN together with OVA induce potent antitumor CTL activities in mice, and the OVA-specific CTL activity is sufficient to inhibit the growth of EG7.OVA tumor cells in mice. Our study also shows that encapsulation of poly-IC or CpG ODN together with antigen in biodegradable nanoparticles is an effective approach for the induction of potent antigen-specific CTL responses.
Figures and Tables
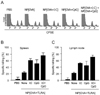 | Figure 1The CTL inducing activities of the nanoparticles. The nanoparticles containing OVA only (NP [OVA]), both OVA and poly-IC (NP [OVA+I:C], or both OVA and CpG ODN (NP[OVA+CpG]) were injected intravenously into tail veins of mice. Seven days later, an in vivo CTL assay was performed in the mice using CFSE-labeled syngeneic target cells. (A) Representative histograms of the slpeen cells of individual mice were shown. The percentages of specific killing of OVA[257-264] peptide-pulsed target cells in the spleens (B) and lymph nodes (C) were graphically represented. |
 | Figure 2The antitumor activities of the nanoparticles. (A) Mice were immunized with the nanoparticles containing bovine serum albumin (BSA) only (NP[BSA]), OVA only (NP[OVA]), both OVA and poly-IC (NP[OVA+I:C], or both OVA and CpG ODN (NP[OVA+CpG]), intravenously into tail veins of the mice. Seven days later, the mice were subcutaneously implanted with EG7.OVA tumor cells (5×105/mouse). Two days later, the mice were again immunized with the same nanoparticles intravenously into tail veins of the mice. The tumor size was measured with a slide caliper and expressed as a tumor index, determined as the square root of (major axis×minor axis). (B) Mice were subcutaneously implanted with the tumor cells, and then mixtures of NP[OVA+I:C] and NP[OVA+CpG] were injected into the tumor mass on 10, 12 and 14 days after the tumor implantation. |
ACKNOWLEDGEMENTS
This study was supported by the research grant of the Chungbuk National University in 2011.
References
1. Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002. 188:51–64.

2. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.

3. Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995. 5:105–109.

4. Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999. 398:77–80.

5. Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001. 19:47–64.

6. Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998. 19:368–373.

7. Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006. 117:78–88.

8. Gerelchuluun T, Lee YH, Lee YR, Im SA, Song S, Park JS, Han K, Kim K, Lee CK. Dendritic cells process antigens encapsulated in a biodegradable polymer, poly(D,L-lactide-co-glycolide), via an alternate class I MHC processing pathway. Arch Pharm Res. 2007. 30:1440–1446.

9. Lee YR, Lee YH, Im SA, Yang IH, Ahn GW, Kim K, Lee CK. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch Pharm Res. 2010. 33:1859–1866.

10. Schliehe C, Redaelli C, Engelhardt S, Fehlings M, Mueller M, van Rooijen N, Thiry M, Hildner K, Weller H, Groettrup M. CD8-dendritic cells and macrophages cross-present poly(D,L-lactate-co-glycolate) acid microsphere-encapsulated antigen in vivo. J Immunol. 2011. 187:2112–2121.

11. Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by Poly (d,l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci. 1998. 87:1421–1427.

12. Venkataprasad N, Coombes AG, Singh M, Rohde M, Wilkinson K, Hudecz F, Davis SS, Vordermeier HM. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine. 1999. 17:1814–1819.

13. Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005. 105:3951–3955.

14. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–347.

15. Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988. 54:777–785.

16. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–347.

17. Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993. 361:359–362.

18. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004. 22:2406–2412.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download