Abstract
Lipocalin-2 (LCN2) is an acute-phase protein induced by injury, infection, or other inflammatory stimuli. LCN2 binds small hydrophobic ligands and interacts with cell surface receptor to regulate diverse cellular processes. The role of LCN2 as a chemokine inducer in the central nervous system (CNS) has been previously reported. Based on the previous participation of LCN2 in neuroinflammation, we investigated the role of LCN2 in formalin-induced nociception and pathological pain. Formalin-induced nociceptive behaviors (licking/biting) and spinal microglial activation were significantly reduced in the second or late phase of the formalin test in Lcn2 knockout mice. Likewise, antibody-mediated neutralization of spinal LCN2 attenuated the mechanical hypersensitivity induced by peripheral nerve injury in mice. Taken together, our results suggest that LCN2 can be therapeutically targeted, presumably for both prevention and reversal of acute inflammatory pain as well as pathological pain.
Inflammation, defined as the immune system response to injury or infection, is an intrinsically beneficial event that leads to the removal of initiating noxious stimuli or offending factors and the restoration of tissue structures and physiological functions. Usually an acute inflammatory response is resolved successfully and the damaged tissue is repaired, rather than becoming persistent and dysfunctional. It may be anticipated, therefore, that the failure of resolving acute inflammation may predispose to autoimmunity, chronic dysplastic inflammation, and excessive tissue damage (1). Acute inflammatory pain hypersensitivity is an outcome of failure in resolving acute inflammation (2,3). Correspondingly, pathological pain occurs in response to extensive, intense, or prolonged tissue or nerve damages and results in extended discomfort and abnormal sensitivity. The neuropathic pain caused by central or peripheral nerve injuries results in the perception of pain even in the presence of normally innocuous stimuli. Pathological pain, or chronic pain, is caused by nerve damages such as nerve compression, nerve trauma, diabetes, infection with herpes zoster virus, autoimmune disease, or cancer (4). Activation of glial cells, especially microglia and astrocytes, in the dorsal horn of the spinal cord and their release of proinflammatory mediators like cytokines, chemokines, prostaglandins, and nitric oxide (NO) have been well documented as the key players in the pathogenesis of pathological pain (5-12). A cascade of events regulated by these mediators results in central sensitization and pain enhancement (13).
Lipocalin-2 (LCN2), which is also known in humans as neutrophil gelatinase-associated lipocalin (NGAL) (14) and in mice as 24p3 (15), is a 25-kDa secretory protein that belongs to the lipocalin family (16). It binds small hydrophobic ligands and interacts with cell surface receptors (24p3R and megalin) to regulate diverse cellular processes. LCN2 is also an acute-phase protein induced by an injury, infection, or other inflammatory stimuli (17). LCN2 has been found to be increased in the prefrontal cortex during inflammatory pain (18) and in the lumbar segment of the spinal cord during peripheral nerve injury-induced neuropathic pain (7). Similarly, LCN2 has been documented to regulate stress-induced changes in spine morphology, neuronal excitability, and anxiety (19). LCN2 is secreted by glial cells (20-22) and acts as a chemokine inducer under neuroinflammatory conditions (23). Recently, LCN2 has been reported to play the critical role in the development of pain hypersensitivity following peripheral nerve injury, suggesting that it mediates neuropathic pain by inducing chemokine expression and subsequent microglial activation (7). Based on these earlier findings, we have speculated that the involvement of LCN2 in the pathogenesis of acute inflammatory pain and the neutralization of LCN2 is a potential pharmacological approach for the prevention and treatment of pathological pain. We have tested these hypotheses using formalin-induced nociceptive behavioral tests and a spared nerve injury (SNI) model. Our findings, based on Lcn2-deficient mice and antibody-mediated neutralization of spinal LCN2, suggest that LCN2 contributes to the pathogenesis of acute inflammatory pain as well as pathological pain.
All experiments were conducted in accordance with the animal care guidelines of the National Institutes of Health. All efforts were made to minimize the number of animals used and animal suffering. Male Lcn2 wild-type (WT, Lcn2+/+) and Lcn2 knockout (KO, Lcn2-/-) mice aged 8~10 weeks were used. LCN2-/- mice were a gift from Dr. Shizuo Akira (Osaka University, Japan). Lcn2+/+ and Lcn2-/- mice were back-crossed for eight to ten generations onto the C57BL/6 background to generate homozygous and heterozygous animals free of background effects on phenotypes, as described previously (23,24). Genotypes were confirmed by polymerase chain reaction (PCR) of genomic DNA (25). The C57BL/6 mice used for breeding were purchased from Samtako (Osan, Korea). Animals were housed under a 12 h light/dark cycle (lights on 07:00~19:00) at a constant ambient temperature of 23±2℃ with food and water provided ad libitum. Animals participated in only one experiment.
Formalin solution in saline (5%; 10 µl) was injected into the plantar surface of the left hindpaw. Mice were then observed for 40 min, and times spent licking or biting injected hindpaws were recorded. The first 10 min post-formalin injection was considered the early/first phase, and the period between 15 min and 40 min the late/second phase (2). Total times spent licking or biting injected paws were measured with a stopwatch by an experimenter blinded to animal genotype or treatment. Activities were recorded over consecutive 5-min periods.
A single intrathecal injection was performed by direct lumbar puncture between the L5 and L6 vertebrae using a 25 µl Hamilton syringe with a 30-gauge needle, as described previously (26). LCN2 antibody was purchased from R&D Systems (Minneapolis, MN) and diluted in phosphate-buffered saline (PBS). Mice were injected intrathecally with 1 µg of LCN2 antibody in a volume of 10 µl. Precise intrathecal localization was confirmed by a tail flick upon penetration. PBS was used as the vehicle control.
The SNI model that mimics human neuropathic pain related to peripheral nerve injury was used as described previously (7,27,28). Briefly, surgery was performed on the left side, defined as ipsilateral side, of mice under 2% isoflurane anesthesia. The contralateral sides were left intact. For the SNI, the three peripheral branches (the sural, common peroneal, and tibial nerves) of the sciatic nerve were exposed, and the tibial and common peroneal branches were ligated with 6-0 silk thread. A ~2 mm segment distal to the ligation site of the two nerves was removed, while the sural nerve was left intact. All wounds were irrigated with sterile saline and closed in layers post-surgery. SNI surgery was performed 30 min after the intrathecal injection of LCN2 antibody or vehicle. The day of surgery was set as day 0.
Mice were allowed to acclimate after arrival in the animal care unit for 1 week, and placed in the test room used for 1 h the day before experiments. Animals were habituated to the test room for at least 1 h before behavioral testing, which was performed by an experimenter unaware of the treatment condition. Mechanical sensitivity was tested using a logarithmic series of calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL), as previously described (29). Briefly, mice were acclimated for 20 min in inverted individual acrylic boxes with wire mesh floors to provide access to the ventral side of the hindpaws. Von Frey hairs were presented perpendicularly to the lateral region of left and right hindpaws, and held in position for approximately 5 s with enough force to cause a slight bend. Two trials per paw were conducted with an interval of at least 3 min. A positive response was defined as abrupt paw withdrawal. Paw withdrawal thresholds (PWTs) were determined by increasing and decreasing forces and estimated using Dixon's up-and-down method (30).
Deeply anesthetized mice were perfused through the aorta with 0.1 M PBS followed by 4% paraformaldehyde (PFA) fixative. Lumbar spinal cord segments (L4-L6) were dissected out, post-fixed in the same PFA fixative overnight, and cryoprotected in 30% sucrose in 0.1 M PBS overnight at 4℃. A cryostat was used to prepare 30 µm sections of the spinal cord kept in 0.1 M PBS. Sections were then blocked with 4% normal serum in 0.3% Triton X-100 for 90 min at room temperature, and then incubated with primary antibodies against Iba-1 (mouse, 1:1,000; Wako, Osaka, Japan) overnight at 4℃. Sections were then incubated with Cy3-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA). Slides were washed, coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and visualized under a fluorescence microscope.
The involvement of LCN2 in acute inflammatory pain was assessed using the formalin test as explained in the experimental timeline (Fig. 1A). An intraplantar injection of formalin resulted in a typical biphasic pain response (2). The first or early phase occurred immediately post-injection due to the direct stimulation of nociceptors, whereas the second or late phase occurred after a short period of quiescence (typically 10~15 min post-injection), during which inflammatory responses took place. This phase reflects central sensitization and continued afferent input (Fig. 1B). No significant difference was observed between Lcn2 KO and WT mice during the early phase, but behavioral responses during the late phase were significantly diminished in Lcn2 KO mice (Fig. 1C). Acute nociceptive pain is caused by the stimulation of peripheral nerve fibers that normally respond only to stimuli approaching or exceeding a harmful intensity. In the formalin-induced acute inflammatory pain model, Lcn2 deficiency did not influence the early phase of formalin-induced behavior [direct chemical effect on peripheral nociceptors (31)]. In contrast to the early phase, late phase-responses [central sensitization in the spinal cord (31)] were significantly diminished in Lcn2 KO animals. In addition, Lcn2 KO mice showed markedly reduced microglial activation in the lumbar spinal dorsal horn during the late phase (Fig. 2). The induction and maintenance of pain hypersensitivity is considered to be due to spinal cord dorsal horn sensitization, also known as central sensitization (32,33). Central sensitization is a specific form of synaptic plasticity in the spinal cord responsible for the enhancement and prolongation of nociceptive responses to both noxious and innocuous stimuli. Growing evidence supports glia as an important source of inflammatory mediators fundamentally involved in the pathogenesis of acute inflammatory and pathological pain (34,35).
Spinal microglia, which also respond to proinflammatory signals released from other non-neuronal cells, amplify the nociceptive response following tissue inflammation or injury (36,37). Acute peripheral nociception is an outcome of the interaction between the peripheral and the central sensitization, which implicates the activation of glial cells (38). Moreover, LCN2 protein has been reported to be secreted by glial cells, and regulate glial cell death/survival, motility, and morphological phenotypes in an autocrine or paracrine manner (20,21). This led us to speculate that acute peripheral nociception is translated into LCN2 overexpression in spinal cord. In the present study, formalin caused spinal microglial activation during the second (late) phase after injection, which was significantly reduced in the Lcn2 KO mice than in the WT mice. These results support the contention that LCN2 contributes to the pathogenesis of acute inflammatory pain by regulating microglial activation in the spinal cord.
On the basis of the findings from the Lcn2-deficient mice and intrathecal injection of LCN2 protein, Jeon et al. have recently reported that the spinal LCN2-chemokine axis may contribute to the pathogenesis of neuropathic pain (7). In reference to these results, we tested the possibility of the neutralization of spinal LCN2 as a therapeutic strategy for the prevention and treatment of pathological pain. Anti-LCN2 antibody at the dose of 1 µg was administered intrathecally to mice 30 min before SNI surgery, and the pain response was measured as described in the experimental timeline (Fig. 3A). In the ipsilateral sides, SNI reduced PWT to force, and this effect was attenuated in the LCN2-neutralized mice than in the control animals at 1~2 days after SNI surgery (Fig. 3B). No significant change in the pain-related behavior was observed in the contralateral sides. As reported previously, LCN2 expressed in the lumbar segment of dorsal spinal cord following SNI surgery might have played an important role in the onset of neuropathic pain (7). Consistent with this notion, the intrathecally administered LCN2 antibody neutralized the expressed spinal LCN2 responsible for the development of the neuropathic pain.
In conclusion, our results suggest that LCN2 participates in behavioral responses to acute inflammatory stimuli and substantiates the critical involvement of LCN2 in the genesis of central sensitization and pathological pain hypersensitivity. These findings indicate that LCN2 can possibly be therapeutically targeted for both prevention and reversal of acute inflammatory and pathological pain.
Figures and Tables
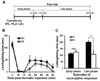 | Figure 1Attenuation of acute inflammatory pain in Lcn2 KO mice. (A) Formalin (5%, 10 µl) is administered intraplantarly to the left hindpaw of mice, then nociceptive behavior is measured as shown in the experimental timeline. (B) The behaviors of wild-type (WT) and Lcn2 KO mice are compared for 40 min after the injection. Times spent licking or biting injected hindpaws are recorded. (C) The first 10 min post-injection is defined as the early phase, and the period between 15 and 40 min post-injection as the late phase. The results are means±SEMs. *p<0.05, n.s.=not significant (n=7~9). |
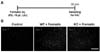 | Figure 2Reduction of microglial activation in Lcn2 KO mice. (A) The lumbar segment (L4-6) of the spinal cord is sampled 30 min after the intraplantar injection of formalin (5%, 10 µl) as shown in the experimental timeline. (B) Photomicrographs showing Iba-1 immunoreactivity in the dorsal horns of the ipsilateral lumbar spinal cords of WT and Lcn2 KO mice. The results are representative of at least three independent experiments. Scale bars=200 µm. |
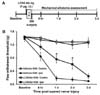 | Figure 3Spinal LCN2 neutralization attenuated SNI-induced pain behavior. (A) LCN2 antibody (0.1 µg/µl, 10 µl), or the same volume of vehicle, is injected intrathecally 30 min before SNI surgery, and the pain test was performed as shown in the experimental timeline. (B) In the ipsilateral side, SNI significantly reduces the paw withdrawal threshold (PWT) to force. The SNI-induced decrease in PWT is significantly attenuated in the LCN2-neutralized mice compared with control animals at 1 day and 2 days after SNI surgery. No significant change in pain-related behavior is observed in the contralateral side. The results are means±SEMs. *p<0.05 versus the vehicle (PBS) group (n=3). |
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A111345), and by the National Research Foundation (NRF) grants funded by the Ministry of Education, Science and Technology (MEST) of the Korean government (2008-0062282).
References
2. Jha MK, Jeon S, Suk K. Glia as a Link between Neuroinflammation and Neuropathic Pain. Immune Netw. 2012; 12:41–47.

3. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: The role and consequences. Neurosci Res. 2013; In press: http://dx.doi.org/10.1016/j.neures.2013.10.004.

5. Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007; 10:1361–1368.

6. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001; 24:450–455.

7. Jeon S, Jha MK, Ock J, Seo J, Jin M, Cho H, Lee WH, Suk K. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. J Biol Chem. 2013; 288:24116–24127.

8. Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013; In press: http://dx.doi.org/10.1016/j.pain.2013.06.022.

9. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009; 10:23–36.

11. Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010; 126:56–68.

12. Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003; 104:655–664.

13. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011; 152:S2–S15.

14. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997; 45:17–23.

15. Hraba-Renevey S, Turler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989; 4:601–608.
16. Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996; 318(Pt 1):1–14.

17. Nilsen-Hamilton M, Liu Q, Ryon J, Bendickson L, Lepont P, Chang Q. Tissue involution and the acute phase response. Ann N Y Acad Sci. 2003; 995:94–108.

18. Poh KW, Yeo JF, Stohler CS, Ong WY. Comprehensive gene expression profiling in the prefrontal cortex links immune activation and neutrophil infiltration to antinociception. J Neurosci. 2012; 32:35–45.

19. Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci U S A. 2011; 108:18436–18441.

20. Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007; 179:3231–3241.

21. Lee S, Park JY, Leem WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009; 29:234–249.

22. Lee S, Lee WH, Lee MS, Mori K, Suk K. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res. 2012; 90:540–550.

23. Lee S, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011; 286:43855–43870.
24. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432:917–921.

25. Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, Fang FC, Weiss G. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009; 114:3642–3651.

26. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980; 67:313–316.

27. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000; 87:149–158.

28. Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lotsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006; 12:1269–1277.

29. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.

30. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462.

31. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987; 30:103–114.

32. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009; 10:895–926.

33. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000; 288:1765–1769.

34. Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010; 229:26–50.

35. Hashioka S, Miyaoka T, Wake R, Furuya M, Horiguchi J. Glia: an important target for anti-inflammatory and antidepressant activity. Curr Drug Targets. 2013; 14:1322–1328.

36. Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012; 26:3103–3117.

37. Vega-Avelaira D, Ballesteros JJ, Lopez-Garcia JA. Inflammation-induced hyperalgesia and spinal microglia reactivity in neonatal rats. Eur J Pain. 2013; 17:1180–1188.

38. Nowak L, Zurowski D, Dobrogowski J, Wordliczek J, Thor PJ. Pentoxifylline modifies central and peripheral vagal mechanism in acute and chronic pain models. Folia Med Cracov. 2012; 52:83–95.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download