Abbreviations
APC
FITC
Foxp3
IFN-γ
LCMV
MHC
PE
PerCP
TCR
TNF-α
Treg
Journal List > Immune Netw > v.13(6) > 1033399
APC
FITC
Foxp3
IFN-γ
LCMV
MHC
PE
PerCP
TCR
TNF-α
Treg
 | Figure 1Detection of LCMV Ag-specific CD4+Foxp3+ iTreg by MHC class II tetramer staining. (A) Diagram of Foxp3GFP and iTreg detection. CD4+Foxp3- T cells sorted from Foxp3GFP knock-in mice were stimulated with anti-CD3/CD28 in the presence or absence of TGF-β for 72 h. The conversion of CD4+Foxp3- Th cells to CD4+Foxp3+ iTregs was evaluated by the expression of GFP fused by Foxp3 molecule. (B) Detection of LCMV-specific CD4+Foxp3+ iTreg specific for three epitopes. Foxp3GFP knock-in mice that had been previously infected with LCMV Armstrong (Arms) were sacrificed to prepare the splenocytes 7 days pi, and the levels of CD4+ T cells specific for different epitopes of LCMV Ag were detected by each MHC class II tetramer staining (I-Ab/DIY, I-Ab/TSA, and I-Ab/TMF). MHC class II tetramer (I-Ab/CLIP) was used for negative control. (C) Opimization of condition to detect LCMV GP66-77-specific CD4+Foxp3+ iTreg. Staining conditions for MHC class II tetramer included I-Ab/CLIP, I-Ab/DIY (42 µg/ml), and I-Ab/DIY (70 µg/ml). After staining with MHC class II tetramer, cells were fixed, stained with anti-CD40 and F4/80 antibody. Some cells were stained by anti-CD4 and F4/80 antibody without fixation. The values in dot-plot denote the average percentage of detected CD4+I-Ab/DIY+ T cells after gating on F4/80-negative cells. (D) Detection of LCMV GP66-77-specific CD4+Foxp3+ iTregs by MHC class II tetramer staining. The splenocytes from LCMV-infected Foxp3GFP knock-in mice were prepared 7 days pi and used for MHC class II tetramer (I-Ab/DIY, 42 and 70 µg/ml) staining to detect CD4+Foxp3+ Treg cells specific for LCMV GP66-77 epitope. The values in dot-plot denote the average percentage of Foxp3+I-Ab/DIY+ gated on CD4+ T cells derived from three independent experiments. |
 | Figure 2Detection of LCMV-specific CD4+ T cells by intracellular CD154 staining. (A) Detection of viable LCMV-specific CD4+ T cells by intracellular CD154 staining. The splenocytes from mice infected with LCMV Arms or Cl13 were prepared 7 days pi and simulated with each specific epitope peptide (LCMV GP66-77, GP126-140, and GP6-20) in presence of PE-conjugated anti-CD154 antibody for 12 h. Splenocytes that was not stimulated with peptide in the presence of PE-conjugated anti-CD154 antibody were used for negative control. The values in dot-plot represent the percentage of CD4+ T cells specific for each LCMV epitope peptide. (B) The profile of IFN-γ and TNF-α expression in LCMV Ag-specific CD4+ T cells detected by intracellular CD154 staining. Following 12 h-stimulation of each epitope peptide in the presence of PE-conjugated CD154 antibody, the cells were stained with anti-CD4 antibody and the expression of IFN-γ and TNF-α in CD154+CD4+ T cells was determined by intracellular cytokine staining. The values in dot-plot represent the average percentage of IFN-γ and TNF-α in CD154+CD4+ T cells. |
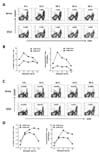 | Figure 3Detection of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg. (A) Detection rate of LCMV GP66-77-specific CD4+ T cells in acute LCMV infection phase, depending on stimulation period. C57BL/6 mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13), and the spelenocytes were prepared 7 days pi and used for stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody for various periods. The values in dot-plot denote the percentage of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide. (B) The frequency and absolute number of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide in acute LCMV infection phase. The graphs represent the average percentage and absolute number of LCMV GP66-77-specific CD4+CD154+ T cells at the indicated stimulation time point. (C) Detection rate of LCMV GP66-77-specific CD4+ T cells in chronic LCMV infection phase, depending on stimulation period. C57BL/6 mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13), and the splenocytes were prepared 44 days pi and used for stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody for various periods. The values in dot-plot denote the percentage of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide. (D) The frequency and absolute number of CD4+CD154+ T cells specific for LCMV GP66-77 epitope peptide in chronic LCMV infection phase. The graphs represent the average percentage and absolute number of LCMV GP66-77-specific CD4+CD154+ T cells at the indicated stimulation time point. |
 | Figure 4Differential expansion of LCMV GP66-77-specific CD4+Foxp3+ iTreg and CD4+Foxp3+ Treg in acute and chronic infection. (A) Frequency of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg in acute and chronic LCMV infection. Foxp3GFP knock-in mice were infected with LCMV Armstrong (Arms) or clone 13 (Cl13) and the splenocytes from LCMV-infected Foxp3GFP knock-in mice were prepared 8 and 35 days pi and used for 12 h-stimulation with LCMV GP66-77 epitope peptide in the presence of PE-conjugated anti-CD154 antibody. The values in dot-plot denote the average of CD154+Foxp3- Th, CD154-Foxp3+ Treg, CD154+Foxp3+ iTreg cells gated on CD4+ T cells (B and C) The frequency and absolute number of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTreg and nonspecific CD4+Foxp3+ Treg cells. The splenocytes of infected Foxp3GFP knock-in mice were prepared 8, 13 and 35 days pi, stimulated with LCMV GP66-77 in the presence of the presence of PE-conjugated anti-CD154 antibody. The bars in graphs represent the average±SD of LCMV GP66-77-specific CD4+CD154+Foxp3+ iTregs (B) and nonspecific CD4+Foxp3+ Treg (C) detected by intracellular CD154 staining in acute and chronic phase. |
APC
FITC
Foxp3
IFN-γ
LCMV
MHC
PE
PerCP
TCR
TNF-α
Treg
Seong Kug Eo 
https://orcid.org/http://orcid.org/0000-0001-9243-4268