Abstract
Ribavirin is an antiviral drug used in combination with pegylated interferon-α (IFN-α) for the treatment of hepatitis C virus (HCV) infection. Recently, ribavirin was reported to inhibit the suppressive activity of regulatory T (Treg) cells. In the present study, we re-evaluated the effect of ribavirin on Foxp3+CD4+CD25+ Treg cells from normal donors. First, we examined the expression of CTLA-4 and CD39, which are known to play a role in the suppressive function of Treg cells. We found that ribavirin treatment did not modulate the expression of CTLA-4 and CD39 in Treg cells. We also studied the effect of ribavirin on Treg cells in the presence of IFN-α; however, the expression of CTLA-4 and CD39 in Treg cells was not changed by ribavirin in the presence of IFN-α. Next, we directly evaluated the effect of ribavirin on the suppressive activity of Treg cells in the standard Treg suppression assay, by co-culturing CFSE-labeled non-Treg CD4+ T cells with purified Treg cells. We found that ribavirin did not attenuate the suppressive activity of Treg cells. Taken together, while ribavirin reversed Treg cell-mediated suppression of effector T cells in the previous study, we herein demonstrate that ribavirin does not impair the suppressive activity of Treg cells.
Ribavirin is an antiviral drug used in combination with pegylated interferon-α (IFN-α) for the treatment of hepatitis C virus (HCV) infection (1,2). Ribavirin is also effective for the treatment of infection with other viruses such as respiratory syncytial virus and Lassa fever virus (3,4). Since the effect of ribavirin on HCV infection was reported for the first time, it has been widely used for the treatment of HCV infection. However, the specific role ribavirin plays in the treatment of HCV infection remains elusive (5).
Several mechanisms have been proposed to explain how ribavirin exerts anti-HCV activity. It was reported that ribavirin inhibits inosine 5'-monophosphate dehydrogenase (6), an important enzyme in guanine nucleotide biosynthesis (7). Ribavirin is also known to inhibit RNA-dependent RNA polymerase of HCV and to induce viral mutagenesis (8). In addition, ribavirin enhances the expression of IFN-stimulated genes by modulating IFN signaling (9,10).
T cell immune responses are also influenced by ribavirin. Initially, it was reported that ribavirin enhances production of T helper 1 (Th1) cytokines such as IFN-γ, TNF-α and IL-2, while suppressing T helper 2 (Th2) cytokines such as IL-4, IL-5 and IL-10 (11,12). However, contradictory results have been reported by other studies (13), thus T cell-regulatory roles of ribavirin are still controversial (14).
Recently, the effect of ribavirin on the suppressive function of regulatory T (Treg) cells was reported (15). In this study, Treg cell clones were generated from patients with chronic HCV infection, and the effect of ribavirin on the Treg cell clones was examined. They found that ribavirin inhibited IL-10 production of the Treg cell clones, and reversed Treg cell-mediated suppression of effector T cells (15).
In the present study, we re-evaluated the effect of ribavirin on Foxp3+CD4+CD25+ Treg cells isolated from normal donors and report here that ribavirin does not impair the suppressive activity of Treg cells.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of normal donors by standard Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation.
The following fluorochrome-conjugated monoclonal antibodies were used for multicolor flow cytometry: anti-CD3-V450 (UCHT1), -V500 (SP34-2) or -APC-H7 (SK7); anti-CD4-FITC or -A700 (RPA-T4); anti-CD25-PerCP-Cy5.5 or -APC (MA251); anti-CD39-PE-Cy7 (eBioA1, eBioscience, San Diego, CA); anti-CD127-Pe-Cy7 or -FITC (HIL-7R-M21); and anti-Fas (CD95)-V450 (DX2) (all from BD Biosciences, San Jose, CA). For intracellular staining, anti-FoxP3-PE (PCH101) (eBioscience) and anti-CTLA-4-APC (BNI3, BD Biosciences) were used. LIVE/DEAD™ fixable aqua dead cell stain kit (Invitrogen, Carlsbad, CA) or 7-aminoactinomycin (7AAD) (BD Biosciences) was used for dead cell gating. Ribavirin was purchased from SIGMA (St. Louis, MO), and IFN-αA/D was purchased from PBL interferon source (Piscataway, NJ).
PBMCs were stained with fluorochrome-conjugated antibodies against surface markers for 30 minutes on ice and then washed. Dead cells were stained by using either Live/Dead Fixable Cell Stain Kit or 7-AAD. For intracellular protein staining, surface-stained cells were permeabilized using FoxP3 Staining Buffer Kit (eBioscience) according to the manufacturer's instructions and further stained for intracellular proteins such as Foxp3 and CTLA-4. Flow cytometry was performed on an LSR II instrument using FACSDiva software (BD Biosciences), and data were analyzed using FlowJo software (Treestar, San Carlos, CA).
Treg cells were isolated from PBMCs using CD4+CD25+ CD127lo/- Treg cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, the non-Treg CD4+ T cells and CD127high cells were first depleted using a cocktail of biotinylated antibodies and anti-biotin microbeads. Then, CD4+CD25+CD127lo/- Treg cells were directly labeled with CD25 microbeads and isolated over a selection column.
For isolation of non-Treg CD4+ T cells, CD25high cells were depleted with CD25 microbeads, and CD4+ T cells were subsequently purified using CD4 microbeads.
The isolated non-Treg CD4+ T cells were labeled with 5µM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen). CFSE-labeled non-Treg CD4+ T cells, serving as responder T (Tresp) cells, were stimulated by soluble anti-CD3 antibody (0.1µg/ml; Beckman Coulter Immunotech, Marseille, France) and anti-CD28 antibody (1µg/ml; BD Biosciences) with or without addition of the isolated Treg cells. After 96 hours of culture, the proliferation of Tresp cells was analyzed by assessing the percentage of CFSElo dividing cells with an LSR II flow cytometer. Dead (7-AAD+) cells were excluded from the analysis. The percentage of suppression was calculated as [1-(% T cell proliferation with Treg cells/% T cell proliferation without Treg cells)]×100.
We isolated PBMCs from whole blood of normal donors and investigated the effect of ribavirin on Foxp3+CD4+CD25+ Treg cells. In the present study, ribavirin was used in a concentration of 2.5µg/ml, since serum concentration of ribavirin in treated patients is approximately 1.5~2.5µg/ml (16,17). First, we examined the expression of CTLA-4 and CD39 in Treg cells because CTLA-4 (18-20) and CD39 (21,22) are involved in the suppressive function of Treg cells and their expression levels correlate with the suppressive activity of Treg cells (18-22). Treg cells express higher levels of CTLA-4 and CD39 than non-Treg CD4+ T cells; however, ribavirin treatment changed the expression of CTLA-4 and CD39 neither in Treg cells nor in non-Treg CD4+ cells (Fig. 1A and B). We also studied the effect of ribavirin on Treg cells in the presence of IFN-α; however, the expression of CTLA-4 and CD39 in Treg cells was not changed by ribavirin in the presence of IFN-α (Fig. 1C and D).
Next, we isolated Foxp3+CD4+CD25+ Treg cells and non-Treg CD4+ T cells from PBMCs of normal donors, then performed the standard Treg suppression assay by co-culture of the isolated Treg cells and CFSE-labeled non-Treg CD4+ T cells. Prior to studying the effect of ribavirin on the results of the standard Treg suppression assay, we evaluated the direct effect of ribavirin on anti-CD3/anti-CD28-mediated proliferation of non-Treg CD4+ T cells. We were able to confirm that there was no direct effect of ribavirin on the proliferation of non-Treg CD4+ responder T cells (Fig. 2A). Finally, we examined the effect of ribavirin on the suppressive activity of Treg cells in the standard Treg suppression assay, and found that ribavirin did not impair the suppressive activity of Treg cells (Fig. 2B and C).
Our current report is contradictory to the results of the previous study which reported the effect of ribavirin on the suppressive activity of Treg cells for the first time (15). In this previous study, ribavirin inhibited IL-10 production of the Treg cell clones and reversed Treg cell-mediated suppression of effector T cells (15). They used Treg cell clones instead of freshly isolated Treg cells, whereas in the present study, we used freshly isolated Treg cells for the standard suppression assay. This difference in the sources of Treg cells might be a cause of the contradictory results. In addition, Treg cells were isolated from normal donors in the present study, while Treg cell clones were derived from patients with chronic HCV infection in the previous study (15). The effect of ribavirin on Treg cells of normal donors might be different from that on Treg cells of chronic HCV patients. This point needs to be clarified in further studies.
In summary, we investigated the effect of ribavirin on Foxp3+CD4+CD25+ Treg cells in the present study. While ribavirin was previously reported to inhibit the suppressive activity of Treg cells, here we demonstrate that ribavirin does not impair the suppressive activity of Treg cells isolated from normal donors. Future studies are warranted to clarify whether the effect of ribavirin on Treg cells is modulated by HCV infection.
Figures and Tables
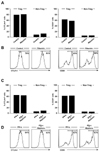 | Figure 1The effect of ribavirin on the expression of CTLA-4 and CD39. (A, B) PBMCs were isolated from whole blood of normal donors and treated with 2.5µg/ml of ribavirin for 3 days. The percentage of CTLA-4+ or CD39+ cells in Foxp3+CD4+CD25+ Treg cells and Foxp3-CD25- non-Treg CD4+ T cells was analyzed by flow cytometry (A). Representative histograms illustrate CTLA-4+ or CD39+ cells within the Foxp3+CD4+CD25+ Treg cell gate (B). (C, D) PBMCs were treated with 2.5µg/ml of ribavirin and 100 U/ml of IFN-α for 3 days. The percentage of CTLA-4+ or CD39+ cells in Foxp3+CD4+CD25+ Treg cells and Foxp3-CD25- non-Treg CD4+ T cells was analyzed by flow cytometry (C). Representative histograms illustrate CTLA-4+ or CD39+ cells within the Foxp3+CD4+CD25+ Treg cell gate (D). The experiment was performed with PBMCs of multiple donors, and data from a single donor is presented as representative data. |
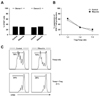 | Figure 2The effect of ribavirin on the suppressive activity of Treg cells. (A) Direct effect of ribavirin on the proliferation of non-Treg CD4+ T cells. Non-Treg CD4+ T cells were labeled with CFSE and stimulated with anti-CD3/anti-CD28 with or without 2.5µg/ml of ribavirin for 4 days, and the percentage of CFSElo dividing cells were analyzed by flow cytometry. (B, C) Foxp3+CD4+CD25+ Treg cells and non-Treg CD4+ T cells were isolated from PBMCs of normal donors, and the standard Treg suppression assay was performed by co-culture of the isolated Treg cells and CFSE-labeled non-Treg CD4+ T cells at the indicated ratio. The percentage of CFSElo dividing cells among non-Treg CD4+ T cells were analyzed by flow cytometry, and the percentage of suppression was calculated as [1-(% T cell proliferation with Treg cells/% T cell proliferation without Treg cells)]×100 (B). Representative histograms illustrate CFSElo dividing cells within the non-Treg CD4+ T cell gate (C). The experiment was performed with PBMCs of multiple donors, and data from a single donor is presented as representative data. |
ACKNOWLEDGEMENTS
This research was supported by the Research Program for New Drug Target Discovery (2010-0030075) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology.
References
1. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001. 358:958–965.

2. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002. 347:975–982.

3. Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma. 2010. 51:1805–1815.

4. McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986. 314:20–26.
5. Paeshuyse J, Dallmeier K, Neyts J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Curr Opin Virol. 2011. 1:590–598.

6. Mori K, Ikeda M, Ariumi Y, Dansako H, Wakita T, Kato N. Mechanism of action of ribavirin in a novel hepatitis C virus replication cell system. Virus Res. 2011. 157:61–70.

7. Shu Q, Nair V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med Res Rev. 2008. 28:219–232.

8. Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006. 16:37–48.

9. Feld JJ, Lutchman GA, Heller T, Hara K, Pfeiffer JK, Leff RD, Meek C, Rivera M, Ko M, Koh C, Rotman Y, Ghany MG, Haynes-Williams V, Neumann AU, Liang TJ, Hoofnagle JH. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology. 2010. 139:154–162.e4.

10. Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011. 53:32–41.

11. Tam RC, Pai B, Bard J, Lim C, Averett DR, Phan UT, Milovanovic T. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999. 30:376–382.

12. Fang SH, Hwang LH, Chen DS, Chiang BL. Ribavirin enhancement of hepatitis C virus core antigen-specific type 1 T helper cell response correlates with the increased IL-12 level. J Hepatol. 2000. 33:791–798.

13. Fujimoto T, Tomimatsu M, Iga D, Endo H, Otsuka K. Changes in the Th1/Th2 ratio during a 24-week course of an interferon alpha-2b plus ribavirin combination therapy for patients with chronic hepatitis C. J Gastroenterol Hepatol. 2008. 23:e432–e437.

14. Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008. 28:1332–1343.

15. Langhans B, Nischalke HD, Arndt S, Braunschweiger I, Nattermann J, Sauerbruch T, Spengler U. Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C. PLoS One. 2012. 7:e42094.

16. Khakoo S, Glue P, Grellier L, Wells B, Bell A, Dash C, Murray-Lyon I, Lypnyj D, Flannery B, Walters K, Dusheiko GM. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998. 46:563–570.

17. Nicot F, Legrand-Abravanel F, Lafont T, Dubois M, Saune K, Pasquier C, Chatelut E, Izopet J. Serum concentrations of ribavirin and pegylated interferon and viral responses in patients infected with HIV and HCV. J Med Virol. 2008. 80:1523–1529.

18. Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006. 177:4376–4383.

19. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008. 322:271–275.

20. Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009. 206:421–434.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download