Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) is a human gut commensal bacteria that causes inflammatory diarrhea and colitis. ETBF also promotes colorectal tumorigenesis in the Min mouse model. The key virulence factor is a secreted metalloprotease called B. fragilis toxin (BFT). BFT induces E-cadherin cleavage, cell rounding, activation of the β-catenin pathway and secretion of IL-8 in colonic epithelial cells. However, the precise mechanism by which these processes occur and how these processes are interrelated is still unclear. E-cadherin form homophilic interactions which tethers adjacent cells. Loss of E-cadherin results in detachment of adjacent cells. Prior studies have suggested that BFT induces IL-8 expression by inducing E-cadherin cleavage; cells that do not express E-cadherin do not secrete IL-8 in response to BFT. In the current study, we found that HT29/C1cells treated with dilute trypsin solution induced E-cadherin degradation and IL-8 secretion, consistent with the hypothesis that E-cadherin cleavage causes IL-8 secretion. However, physical damage to the cell monolayer did not induce IL-8 secretion. We also show that EDTA-mediated disruption of E-cadherin interactions without E-cadherin degradation was sufficient to induce IL-8 secretion. Finally, we determined that HT29/C1 cells treated with LiCl (β-catenin activator) induced IL-8 secretion in a dose-dependent and time-dependent manner. Taken together, our results suggest that BFT induced IL-8 secretion may occur by the following process: E-cadherin cleavage, disruption of cellular interactions, activation of the β-catenin pathway and IL-8 expression. However, we further propose that E-cadherin cleavage per se may not be required for BFT induced IL-8 secretion.
Enterotoxigenic Bacteroides fragilis (ETBF) is an intestinal bacteria that has been associated with inflammatory bowel disease and colorectal cancer in humans (1,2). ETBF also cause diarrhea and colitis in both livestock and laboratory animals (3-7). In the Min mouse model, ETBF promotes colonic tumorigenesis via the Th17/IL-23 pathway (8). The only known virulence factor specific for ETBF is the secreted 20 kDa metalloprotease called B. fragilis toxin (BFT) (9,10). Addition of purified BFT to colonic epithelial cell lines induces several distinct changes. These include ectodomain cleavage of E-cadherin, morphological "rounding" of cells and secretion of IL-8 (11-14). E-cadherin is a 120 kDa type I transmembrane protein essential to the formation of intercellular adhesion of adjacent epithelial cells (15). The cytoplasmic domain of E-cadherin is bound to β-catenin, which in turn associates with α-catenin and cytoskeletal actin (16). These associations result in formation of a stable epithelial monolayer which provides a protection barrier against infiltration of external insults. The loss of this epithelial integrity results in inflammatory disorders including colitis. BFT induces rapid cleavage of the extracellular domain of E-cadherin which result in cell rounding and loss of epithelial integrity. Subsequent E-cadherin degradation by γ-secretase releases the bound β-catenin and nuclear translocation of β-catenin activates the β-catenin-TCF-dependent pathway (17).
To date, several cytokines and chemokines have been identified to be secreted in response to BFT treatment of intestinal epithelial cells: TGF-β, ENA-78, GRO-α, MCP-1 and IL-8 (13,14,18). IL-8 is a potent inflammatory chemokine that is quickly secreted in response to microbial insults and functions to recruit neutrophils to sites of damage. IL-8 induction can occur through activation of the NF-κB and MAPK pathways. Using hepatoma cells, Levy et al. found that stimulation of the β-catenin pathway induces expression of IL-8 due to the presence of a unique consensus Tcf/Lef site that is critical for IL-8 activation by β-catenin (19). Taken together, we propose a model for BFT-induced IL-8 secretion in which the enzymatically active BFT induces E-cadherin degradation, which results in release of the bound β-catenin that in turn translocate into the nucleus and actives IL-8 expression. In this study, we present data suggesting that activation of the β-catenin pathway in the colonic epithelial cell line by disruption of the E-cadherin junction is sufficient to induce IL-8 secretion.
The human colonic epithelial cells line (HT29/C1) was originally obtained from Dr. Daniel Louvard, Institut Pasteur, Paris, France). HEK293/17 cells were purchased from ATCC. Cells were cultured in 10% FBS-DMEM containing gentamicin (100 ug/ml) and penicillin/streptomycin. All cell culture reagents were purchased from GIBCO BRL Life Technologies (Rockville, MD, USA). Cells were grown to subconfluent monolayers (~70%) in 6-well plates. The cells were then washed with serum-free DMEM three times and then cultured with 3 ml of serum-free DMEM containing purified BFT (100 ng/ml), EDTA (Sigma-Aldrich, USA), NaCl (Sigma-Aldrich, USA), LiCl (Sigma-Aldrich, USA), DSS (MW 30,000~45,000; MP Biochemicals, USA), 0.05% trypsin solution (Gibco, USA) or recombinant human IL-1β (50 ng/ml)(R&D Systems, USA) for 24 hr. To induce physical damage to cells, the subconfluent monolayer was scratched full length with a 1 ml pipette tip either 5 or 20 times.
For IL-8 ELISA, cell culture supernatants were harvested, clarified by centrifugation and stored at -20℃ until analyzed by an IL-8 ELISA kit (R&D Systems, USA). Western blot analysis was performed as previously described (4). In brief, cells were washed two times with ice-cold PBS and lysed on ice for 15 min in RIPA buffer (Sigma-Aldrich, USA) containing protease inhibitors (Roche, USA). Clarified lysates were stored at -20℃ until analyzed by Western blot analysis using monoclonal antibodies against the extracellular domain of E-cadherin (H108, Santa Cruz Biotechnology, USA) or β-actin (Sigma-Aldrich, USA). Lysates were electrophoresed under reducing conditions on 12% SDS-PAGE gels and then transferred to 0.22-µm nitrocellulose membranes. Appropriate HRP-conjugated secondary antibodies and SuperSignal West Pico chemiluminescent substrates (Pierce, USA) were used to develop the bands on X-ray film.
Statistical analysis and graphic presentation were performed using GraphPad Prism 5. p-values were calculated using Anova. Values are shown as mean and standard error of mean (SEM). Data were collected from 3~5 independent experiments. A p-value<0.05 was considered statistically significant.
As previously reported, BFT induces IL-8 secretion in the colonic epithelial cell line HT29/C1 which expresses E-cadherin (Fig. 1A). The induction of IL-8 secretion is considered to be due to cleavage of E-cadherin which releases the intracellular bound β-catenin leading to IL-8 secretion. Cells that do not express E-cadherin such as HEK293/17 cells do not secrete IL-8 in response to BFT treatment although these cells do have the capacity to release potent levels of IL-8 in response to inflammatory cytokine stimulation such as IL-β (Fig. 1A). These results as well as results from other studies suggest that induction of IL-8 by BFT requires E-cadherin cleavage (4). If induction of IL-8 is due to disruption of the E-cadherin junction by BFT mediated cleavage of E-cadherin, then enzymatic cleavage of the E-cadherin ectodomain by other enzymes should also induce IL-8. To test this hypothesis, HT29/C1 cells were treated with dilute trypsin for 24 hr and the supernatant assessed for IL-8 secretion by ELISA. We found that trypsin treated HT29/C1 cells showed a dramatic loss of the full length 120 kDa E-cadherin protein and become refractile indicating a loss of cell-to-cell contact (data not shown). In trypsin treated cells, IL-8 secretion was induced in a trypsin concentration dependent manner suggesting that E-cadherin cleavage resulted in IL-8 induction (Fig. 1B). Physical damage of the cellular monolayer was insufficient to induce IL-8 secretion as scratching the subconfluent monolayers with a sterile pipette tip either 5 times or 20 times did not induce IL-8 secretion (Fig. 1B).
The results from the BFT treatment and trypsin treatment strongly suggest that cleavage of E-cadherin is the underlying reason for IL-8 induction. Homophilic E-cadherin interactions between adjacent epithelial cells require the presence of calcium to mediate a stable interaction. Addition of the calcium-chelating EDTA is known to disrupt this interaction without inducing E-cadherin cleavage. We examined if disruption of the E-cadherin homophilic interaction was sufficient to induce IL-8 addition. HT29/C1 cells were treated with different doses of EDTA (0, 1, 2.5, 5 mM) for 24 hr and the secreted IL-8 examined by ELISA. We found an EDTA dose-dependent increase in IL-8 secretion (Fig. 2A). The addition of EDTA did not change any noticeable changes in protein levels of the full length E-cadherin and no evidence of E-cadherin cleavage as determined by Western blot analysis (Fig. 2B). Similarly, we found that physical damage of the HT29/C1 cell monolayer using a pipette tip did not induce E-cadherin cleavage (Fig. 2B). These results suggest that disruption of E-cadherin interaction without E-cadherin cleavage is sufficient to induce IL-8 secretion.
The promoter of the IL-8 gene contains a binding site for the β-catenin/TCF complex (19). This observation suggests that activation of the β-catenin pathway may be sufficient to induce IL-8 secretion in HT29/C1 cells. To determine if HT29/C1 cells can secrete IL-8 through activation of the β-catenin pathway, we treated HT29/C1 cells with LiCl which is known to stimulate the β-catenin pathway (19,20). HT29/C1 cells were treated with different concentrations of LiCl for 24 hr and IL-8 secretion determined by ELISA. We found a LiCl dose-dependent increase in IL-8 secretion beginning at 10 mM which then peaked at 50 mM. At 100 mM LiCl, IL-8 secreted was completely inhibited to background levels suggesting an optimal dose of 50 mM (Fig. 3A). Equal concentrations of NaCl served as a control for LiCl treatment which did not induce IL-8 secretion at all concentrations used (Fig. 3A). Next, we conducted a time course experiment with LiCl by treating HT29/C1 cells with 50 mM LiCl for 2, 4, 6 or 8 hrs and measured IL-8 by ELISA. We found an increase in IL-8 secretion beginning at 2 hr post-treatment and thereafter gradually increased over time (Fig. 3B). These results suggest that activation of the β-catenin pathway is sufficient to induce IL-8 secretion in the HT29/C1 cell line.
ETBF has been suggested to contribute to development of colon cancers (8). Considering that IL-8 expression correlates with induction and progression of colorectal cancer, it would be important to understand the precise mechanism by which BFT induces IL-8 secretion (21). Taken together, our results suggest that in the HT29/C1 cell line, BFT induces IL-8 secretion by disruption of E-cadherin junctions and changes in cellular contacts which then lead to activation of the β-catenin pathway. BFT is known to induce E-cadherin cleavage and IL-8 secretion. However, it is not clear if E-cadherin cleavage is required for IL-8 secretion. Our data, raise the possibility that E-cadherin disruption and therefore cell-cell dissociation is sufficient to induce IL-8 secretion. We suggest that E-cadherin cleavage per se may not be required for BFT induced IL-8 secretion.
Figures and Tables
 | Figure 1Induction of IL-8 secretion by various stimuli. (A) HT29/C1 cells or HEK293/17 cells were cultured with BFT (100 ng/ml) or IL-1β (50 ng/ml) for 24 hr and IL-8 secretion assessed by ELISA. (B) HT29/C1 cells were cultured with diluted trypsin solution (1/100, 1/1,000) or dextran sulfate sodium (DSS) for 24 hr and IL-8 secretion assessed by ELISA. To induce physical damage, the subconfluent cell monolayer was scratched in a line with a pipette tip either 5 or 20 times. Asterisks indicate p<0.05. Data are shown as mean and SEM. |
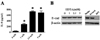 | Figure 2Disruption of E-cadherin interaction but not physical damage induces IL-8 secretion. Subconfluent HT29/C1 cells were cultured with EDTA, scratched (20 times) or treated with purified BFT (100 ng/ml) for 24 hr. (A) IL-8 secretion. (B) Western blot analysis of E-cadherin and β-actin. Cells were treated with Asterisks indicate p<0.05. Data are shown as mean and SEM. |
ACKNOWLEDGEMENTS
This study was supported in part by a New Investigator Start-Up Grant from Yonsei University at Wonju (#2011-5-5018) to Ki-Jong Rhee.
References
1. Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000; 6:171–174.

2. Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006; 12:782–786.
3. Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001; 39:1737–1746.
4. Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad AR, Gan CM, Housseau F, Sears CL. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009; 77:1708–1718.

5. Yim S, Gwon SY, Hwang S, Kim NH, Jung BD, Rhee KJ. Enterotoxigenic Bacteroides fragilis causes lethal colitis in Mongolian gerbils. Anaerobe. 2013; 21:64–66.

6. Rabizadeh S, Rhee KJ, Wu S, Huso D, Gan CM, Golub JE, Wu X, Zhang M, Sears CL. Enterotoxigenic Bacteroides fragilis: A potential instigator of colitis. Inflamm Bowel Dis. 2007; 13:1475–1483.
7. Nakano V, Gomes DA, Arantes RM, Nicoli JR, Avila-Campos MJ. Evaluation of the pathogenicity of the Bacteroides fragilis toxin gene subtypes in gnotobiotic mice. Curr Microbiol. 2006; 53:113–117.

8. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009; 15:1016–1022.

9. Franco AA, Mundy LM, Trucksis M, Wu S, Kaper JB, Sears CL. Cloning and characterization of the Bacteroides fragilis metalloprotease toxin gene. Infect Immun. 1997; 65:1007–1013.

10. Moncrief JS, Obiso R Jr, Barroso LA, Kling JJ, Wright RL, Van Tassell RL, Lyerly DM, Wilkins TD. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect Immun. 1995; 63:175–181.

11. Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA. 1998; 95:14979–14984.

12. Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004; 72:5832–5839.

13. Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001; 123:421–427.

14. Sanfilippo L, Li CK, Seth R, Balwin TJ, Menozzi MG, Mahida YR. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-b) by human colonic epithelial cells. Clin Exp Immunol. 2000; 119:456–463.

15. Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007; 178:323–335.

16. Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995; 92:5067–5071.

17. Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and {gamma}-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007; 120:1944–1952.

18. Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 2005; 35:2648–2657.

19. Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002; 277:42386–42393.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download