Abstract
Dectin-1, which specifically recognizes β-glucan of fungal cell walls, is a non-Toll-like receptor (TLR) pattern recognition receptor and a representative of C-type lectin receptors (CLRs). The importance of Dectin-1 in innate immune cells, such as dendritic cells and macrophages, has previously been well studied. However, the function of Dectin-1 in B cells is very poorly understood. To determine the role of Dectin-1 in B cell activation, we first investigated whether mouse B cells express Dectin-1 and then assessed the effect of Dectin-1 stimulation on B cell proliferation and antibody production. Mouse B cells express mRNAs encoding CLRs, including Dectin-1, and surface Dectin-1 was expressed in B cells of C57BL/6 rather than BALB/c strain. Dectin-1 agonists, heat-killed Candida albicans (HKCA) and heat-killed Saccharomyces cerevisiae (HKSC), alone induced B cell proliferation but not antibody production. Interestingly, HKSC, HKCA, and depleted zymosan (a selective Dectin-1 agonist) selectively enhanced LPS-driven IgG1 production. Taken together, these results suggest that, during fungal infection, β-glucan-stimulated Dectin-1 may cooperate with TLR4 to specifically enhance IgG1 production by mouse B cells.
During microbial infection, immune cells recognize various common microbial shapes (named pathogen-associated molecular patterns, PAMPs) through their own receptors (named pattern recognition receptors, PRRs) to elicit innate and adaptive immune responses against various pathogens (1,2). Cell-associated PRRs include transmembrane receptors [Toll-like receptors (TLRs) and C-type lectin receptors (CLRs)] and cytoplasmic receptors (NOD-like receptors and RIG-I-like receptors). Among them, CLRs primarily mediate immune responses for the infection of fungal pathogens (e.g. Candia albicans, Aspergillus fumigatus, and Cryptococcus neoformans) (3-5). Several CLRs, such as Dectin-1, Dectin-2, mannose receptor, DC-SIGN, and Mincle, can induce intracellular signaling upon recognition of fungal cell wall PAMPs, α- and β-glucan (a polymer of glucose), mannans (chains of N- or O-linked mannose molecules), and chitin (a polymer of N-acetylglucosamine) (5,6). β-glucan is a well characterized fungal PAMP and can comprise up to 50% of the dry weight of fungal cell wall. Fungal PAMPs recognition by CLRs stimulates their uptake and killing by phagocytes and induces inflammatory cytokines and chemokines. In addition, fungal PAMPs-simulated phagocytes produce other cytokines, IL-1β, IL-6, IL-12, and IL-23, to drive the development of protective Th1/Th17 and invariant natural killer T cells responses (5-7).
Dectin-1, which specifically binds to β-glucan, is the first and the best-studied non-TLR PRR (8). Dectin-1 is expressed primarily by myeloid cells, such as macrophages, neutrophils and dendritic cells (DC) as well as other cell types, human B cells and eosinophils (9). In vitro characterization with isolated primary cells, such as macrophages and DC, and in vivo studies with Dectin-1 deficient mice have demonstrated that, during fungal infection, Dectin-1 is essential for inducing phagocytosis and killing of fungi and also for leading the induction of numerous cytokines and chemokines to promote antifungal immunity (9-11). Thus, the function of CLRs, including Dectin-1, for fungal immunity has been extensively studied in innate immune cells (especially macrophages and DC). Antifungal antibodies are very important to protect hosts against pathogenic fungi. (12-16). However, the mechanism of B cell humoral response and the role of Dectin-1 of B cells against fugal infections are still under the veil.
Mature B cells can be activated and differentiated into plasma cells to produce five different isotype antibodies (IgM, IgD, IgG, IgE, and IgA) upon stimulation with antigens (including PAMPs), cytokines, and T cells help (CD40-C40L interaction). The components of most fungal cell wall recognized by CLRs are non-proteins, such as β-glucans, mannans, and chitin. Hence, antibody production through thymus (T)-independent B cell response may be more important than T-dependent antibody response during fungal infections. To evaluate the direct effects of Dectin-1 ligands on B cells in vitro, we used not only total cells but also purified B cells from mouse spleen in this study. Here, we found that production of IgG1 can be directly enhanced by stimulation of Dectin-1, which expressed on mouse B cells, in cooperation with TLR4 triggering.
C57BL/6 and BALB/c mice were purchased from Damool Science (Daejeon, Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber. Eight- to twelve-week-old mice were used, and animal care was in accordance with the institutional guidelines of the Institutional Animal Care and Use Committee of Konyang University.
Mouse spleen B cells were purified by positive selection of B220+ cells using anti-B220 microbeads or by depletion of CD43+ cells using anti-CD43 microbeads and high-gradient magnetic cell separation (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's instructions. Briefly, C57BL/6 or BALB/c mouse spleen cell suspensions were washed with HBSS (WelGENE, Daegu, Korea) and treated with 0.83% ammonium chloride to lyse red blood cells. Spleen cells were treated with either anti-mouse B220 microbeads or anti-mouse CD43 microbeads and separated by LS column and MACS Separator (Miltenyi Biotec). The purity of B cells (≥98%) assessed by FACSCalibur (BD Biosciences, San Jose, CA, USA) following staining of the cells with anti-CD43 FITC (eBioscience, San Diego, CA, USA) and/or anti-B220 PE (BD Biosciences). The sIgM+ mouse B lymphoma line L10A6.2 was provided by Dr. J. Stavnezer (University of Massachusetts Medical School, Worcester, MA, USA). The macrophage cell line RAW264.7 was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37℃ in a humidified CO2 incubator (Forma Scientific, Marietta, OH, USA) in RPMI-1640 medium (WelGENE) supplemented with 10% fetal bovine serum (PAA Laboratories, Etobicoke, ON, Canada). Mouse total spleen cells or purified B cells were stimulated with heat-killed Candida albicans (HKCA, 1×107 cells/ml, InvivoGen, San Diego, CA, USA), heat-killed Saccharomyces cerevisiae (HKSC, 1×107 cells/ml, InvivoGen), depleted zymosan (dzn, 100 µg/ml, InvivoGen), LPS (1 µg/ml, InvivoGen) and F(ab')2 fragment anti-mouse IgM (5 µg/ml, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Surface Dectin-1 expression was analyzed by flow cytometry (FACSCalibur) after staining with antimouse Dectin-1 FITC or isotype (IgG2b) control FITC (AbD Serotec, Raleigh, NC, USA).
RNA preparation, reverse transcription, and PCR were performed as previously described (17). The following PCR primers were synthesized by Bioneer (Daejeon, Korea): Dectin-1, forward 5'-CAAGTGCTCTGCCTACCTAG-3', reverse 5'-CACCATCTTTATATTCTCACATAC-3' (This primer set amplifies two different spliced isoforms. product size, 700 and 795 bp) (18); Dectin-2, forward 5'-ACCCCTGACCTTCTGAACATACAC-3', reverse 5'-TGAGCCCCCATCTGAACACA-3' (This primer set amplifies two different spliced isoforms. product size, 263 and 368 bp) (19); Mincle, forward 5'-GCTCCAGCAGGGAACAATAG-3', reverse 5'-GCCCTTTGATGGAATTCAGA-3' (product size, 185 bp); mannose receptor, forward 5'-CCCTCTGGTGAACGGAATGATTGTGTAG-3', reverse 5'-GCTGCAACGCCGGCACCTATCAC-3' (product size, 248 bp); and β-actin, forward 5'-CATGTTTGAGACCTTCAACACCCC-3', reverse 5'-GCCATCTCCTGCTCGAAGTCTAG-3' (product size, 320 bp). All reagents for RT-PCR were purchased from iNtRON Biotechnology (Seongnam, Korea). PCR for β-actin was performed in parallel to normalize cDNA concentrations within each set of the samples. Aliquots of the PCR products were resolved by electrophoresis on 2% agarose gels.
Cell proliferation was determined by EZ-Cytox cell viability assay kit (Daeil Lab Service Co, Seoul, Korea) as previously described (20). Briefly, 20 µl of EZ-Cytox kit reagent was added to each cell cultured well of a 96-well microplate and then incubated at 37℃ in a humidified CO2 incubator for 3 h. After incubation, optical density (OD) was measured at a wavelength of 450 nm using an Absorbance Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Antibodies produced in B cell cultures were detected by using isotype-specific ELISA. Affinity purified anti-isotype specific antibodies were added at 1.2 µg/ml in 0.05 M sodium bicarbonate buffer (pH 9.5) to 96-well U bottomed polyvinyl microplates (Falcon, Becton Dickinson & Co., Oxnard, CA, USA). Plates were washed with PBS containing 0.05% Tween-20 (PBST) followed by overnight incubation at 4℃, and blocked for 1 h with 0.25% BSA solution. After washing, 50 µl of standard myeloma proteins and culture supernatants were added to each well and incubated for 1 h at 37℃. After washing, horseradish-peroxidase (HRPO) conjugated anti-isotype specific antibodies (Southern Biotechnology, Birmingham, AL, USA) were added to each well and incubated for 1h. Plates were then washed, and TMB substrate (BD Biosciences) was added. After incubation, 0.05 M sulfuric acid was added to each well, and colorimetric reaction was measured at 450 nm with an Absorbance Microplate Reader.
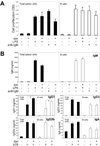
Dectin-1 is widely expressed in innate immune cells, such as DCs, macrophages/monocytes, and neutrophils of both mouse and human (8). In addition, Brown's group reported that human B cells also express Dectin-1 (21), but Dectin-1 expression was not detected in mouse B cells (22). However, more recently, it has been reported that curdlan, a selective Dectin-1 agonist, directly activates mouse B cells and induces IgM production (23). This result strongly suggests that mouse B cells also express Dectin-1. Therefore, we first determined whether Dectin-1 is expressed in mouse B cells to explore the role of Dectin-1 in the cells. As shown in Fig. 1A, purified B cells from BALB/c and C57BL/6 mice as well as mouse B lymphoma, L10A6.2, express Dectin-1 mRNA. These cells also express mRNAs of other CLRs, including Dectin-2, Mincle, and mannose receptor. RAW264.7 mouse macrophage cell line was used as a positive control for CLRs expression. Also, we observed that surface Dectin-1 is expressed in B220+ B cells from the spleen of C57BL/6 and L10A6.2 mouse B cell lymphoma (Fig. 1B). Thus, mouse B cells express Dectin-1 mRNA and surface Dectin-1. To evaluate the role of Dectin-1 in mouse B cell activation in vitro, we used purified B cells from the C57BL/6 mouse spleen in this study.
As we mentioned above, Dectin-1 is a specific receptor for β-glucan, which is found in the cell walls of fungi, including the yeasts Candida albicans and Saccharomyces cerevisiae. Therefore, we investigated the effects of two known Dectin-1 agonists, heat-killed C. albicans (HKCA) and heat-killed S. cerevisiae (HKSC), on proliferation of total spleen cells and antibody production. Signaling for B cell activation and differentiation is provoked by various stimulations, such as B cell receptor (BCR) cross-linking by antigen binding, TLRs-PAMPs engagement, CD40-CD40L interaction, and cytokines, e.g. BAFF and APRIL. Many reports have demonstrated the cooperation between Dectin-1 and TLRs to induce immune activation in innate immune cells (24-28). For instance, Dectin-1 synergizes with TLR2 and TLR4 signals in macrophages/monocytes and DC, and it promotes Th1 and Th17 responses to activate antifungal host defense (29,30), and the combination of anti-hDectin-1 and TLR agonists further enhance the activation of DCs (31). In this study, we attempted to determine whether Dectin-1 cross-talks with TLR4, a receptor for mouse B cell mitogen LPS, and BCR to induce mouse B cell activation. As shown in Fig. 2A, HKCA or HKSC alone induced mouse spleen cell proliferation. Meanwhile, the two dectin-1 agonists did not further increase LPS- or anti-IgM (BCR cross-liking)-induced B cell proliferation but decreased LPS-induced proliferation at 3 days of culture. Interestingly, HKCA or HKSC alone did not induce IgM production but decreased LPS-induced IgM production. Similarly, treatment with anti-IgM did not induce IgM production, although it effectively induced B cell proliferation. Other isotypes were also not induced by HKCA or HKSC alone, or anti-IgM (data not shown). These results suggest that Dectin-1 or BCR stimulation alone is not enough to induce B cell differentiation and produce antibodies, even if they provide signals for cell proliferation. On the other hand, it was found that HKCA and HKSC augmented LPS-driven IgG1 secretion, whereas other isotypes, i.e. IgM, IgG3, IgG2b, and IgA, were decreased (Fig. 2A). In the same manner as total spleen cells, when we tested with purified B cells, HKCA decreased LPS-induced B cell proliferation but selectively and markedly enhanced LPS-driven IgG1 production (Fig. 2B). These results indicate that Dectin-1 stimulation by HKCA and HKSC collaborates with TLR4 signaling to enhance IgG1 production.
In general, HKCA and HKSC are used as Dectin-1 agonists as we tested above. However, it is possible that HKCA and HKSC contain other components to activate other PRRs except Dectin-1 in mouse B cells. Zymosan is a particulate β-glucan prepared from cell wall of S. cerevisiae and stimulates both Dectin-1 and TLR2 in macrophages, while depleted zymosan (dzn) activates Dectin-1 but not TLR2 (24,32). Therefore, we next used a more selective Dectin-1 agonist dzn to evaluate its specific effect on Dectin-1 for B cell proliferation and antibody production. Unlike HKCA and HKSC, dzn alone did not induce cell proliferation in culture of either total spleen cells or purified B cells (Fig. 3A). This result implies that there may be significant differences between HKCA/HKSC and dzn in intrinsic characteristics for the induction of cell proliferation. In addition, dzn enhanced neither LPS- nor anti-IgM-induced B cell proliferation. Like HKCA and HKSC, dzn augmented LPS-driven IgG1 production by either total spleen cells or purified B cells, but not other isotypes, IgM, IgG3, IgG2b, and IgA (Fig. 3B). Collectively, overall patterns of cell proliferation and antibody production by Dectin-1 stimulations were the same in both total spleen cells and purified B cells. Thus, Dectin-1 agonists may directly drive the differentiation of TLR4-stimulated B cells and induce differential antibody production.
In summary, this study showed for the first time that mouse B cells express Dectin-1 and that stimulation of the Dectin-1 can collaborate with LPS to selectively induce IgG1 secretion. To further confirm these results, we are currently investigating the effect of curdlan, another selective Dectin-1 agonist, on mouse B cell proliferation and antibody production because it has already been proved that curdlan alone induces mouse B cell activation (23). In addition, to further explore the mechanism by which Dectin-1 cross-talks with TLR4 signal to enhance IgG1 production, we are studying the effects of Dectin-1 agonists on expression of germline γ1 transcript, which is prerequisites for subsequent IgG1 isotype switching. In conclusion, the results of this study suggest a novel humoral response during fungal infection through which direct stimulation of Dectin-1 may cause drives B cells to selectively enhance IgG1 antibody production against fungi (e.g. C. albicans) in cooperation with TLR4. Recently, both the morbidity of mycoses by fungal opportunistic infection and the mortality by mycoses have consistently increased in immune-compromised patients. Therefore, there is the need to develop fungal vaccines because current antifungal therapy is toxic and ineffective (33). A clear understanding of the mechanism of antifungal humoral immunity, including antibody production by B cells, would foster the development of vaccines and advance the development of biological therapeutics to modulate the host's immune response during fungal infection.
Figures and Tables
Figure 1
Expression of Dectin-1 in mouse B cells. (A) mRNA expression of Dectin-1, Dectin-2, Mincle and mannose receptor. Total RNAs were isolated from purified mouse B cells and the indicated cell lines (L10A6.2 and RAW264.7), and levels of Dectin-1, Dectin-2, Mincle and mannose receptor (MR) mRNA were measured by RT-PCR. (B) Expression of surface Dectin-1 in mouse B cells. Isolated total mouse spleen cells from BALB/c and C57BL/6 and the indicated cell lines were stained with anti-mouse Dectin-1 FITC and anti-B220 PE, and populations of Dectin-1+ cells were determined by flow cytometric analysis.

Figure 2
Effects of Dectin-1 agonists, HKCA and HKSC, on B cell proliferation and antibody production. (A) Total spleen cells isolated from C57BL/6 were stimulated HKCA (1×107 cells/ml), HKSC (1×107 cells/ml), LPS (1 µg/ml) and anti-IgM (5 µg/ml). (B) Purified B cells from spleen of C57BL/6 were stimulated as in (A). After 2 and 3 days of culture, cell proliferation (OD) was measured by EZ-Cytox cell viability assay. Data are averages of duplicate samples with ranges (bars). After 7 days of culture, supernatants were harvested, and levels of Igs production were determined by isotype-specific ELISA. Data represent means±SEM of triplicate samples. *p<0.05.

Figure 3
Effect of the selective Dectin-1 agonist, depleted zymosan, on B cell proliferation and antibody production. Isolated total spleen cells and purified spleen B cells from C57BL/6 were stimulated with depleted zymosan (dzn, 100 µg/ml), LPS (1 µg/ml) and anti-IgM (10 µg/ml). (A) After 2 days of culture, cell proliferation was measured by EZ-Cytox cell viability assay. Data are averages of duplicate samples with ranges (bars). (B) After 7 days of culture, supernatants were harvested, and levels of Igs production were determined by isotype-specific ELISA. Data represent means±SEM of triplicate samples. *p<0.05.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (NRF-2013R1A1A2013036) and partially supported by the grant of Myunggok Medical Research Institute in Konyang University. B.-S.S. is an awardee of full research scholarships provided by Graduate School of Konyang University.
References
2. Michallet MC, Rota G, Maslowski K, Guarda G. Innate receptors for adaptive immunity. Curr Opin Microbiol. 2013; 16:296–302.

3. Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008; 16:27–32.

4. Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011; 29:1–21.

5. Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012; 13:817–822.

6. Wuthrich M, Deepe GS Jr, Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012; 30:115–148.

8. Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006; 6:33–43.

9. Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011; 14:392–399.

10. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007; 8:31–38.

12. Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002; 16:791–802.

13. Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005; 202:597–606.

14. Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006; 91:1–44.

15. McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest. 2010; 120:1355–1361.

16. Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012; 11:447–456.

17. Park SR, Kim PH, Lee KS, Lee SH, Seo GY, Yoo YC, Lee J, Casali P. APRIL stimulates NF-kappaB-mediated HoxC4 induction for AID expression in mouse B cells. Cytokine. 2013; 61:608–613.

18. Yang Z, Marshall JS. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology. 2009; 214:321–330.

19. Ariizumi K, Shen GL, Shikano S, Ritter R 3rd, Zukas P, Edelbaum D, Morita A, Takashima A. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem. 2000; 275:11957–11963.

20. Kim YH, Lee SH, Yoo YC, Lee J, Park JH, Park SR. Kinetic analysis of CpG-Induced mouse B cell growth and Ig production. Immune Netw. 2012; 12:89–95.

21. Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005; 35:1539–1547.

22. Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002; 169:3876–3882.

23. Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009; 183:8061–8067.

24. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003; 197:1107–1117.

25. Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004; 112:521–530.

26. Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008; 38:500–506.

27. Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 2009; 39:1379–1386.

28. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–650.

29. LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007; 8:630–638.

30. Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008; 10:2058–2066.

31. Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O'Bar A, Clayton S, Palucka AK, Zurawski G, Banchereau J, Oh S. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010; 185:3504–3513.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download