Abbreviations
T1D
TLR
DC
NOD
DPP4
Mφ
DAMP
APC
CRAMP
GLP-1
iPSc
Journal List > Immune Netw > v.13(5) > 1033387
T1D
TLR
DC
NOD
DPP4
Mφ
DAMP
APC
CRAMP
GLP-1
iPSc
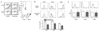 | Figure 1TLR2 tolerance induced by prolonged treatment with Pam3CSK4 in vivo. (A) When CD4+ diabetogenic T cells from BDC2.5/NOD mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) and transferred into control recipient NOD mice, CD4+ T cell proliferation measured by analyzing CFSE dilution by FACS analysis gated on CD4+ and Vβ4+ cells was observed specifically in the pancreatic lymph nodes but not in the mesenteric lymph nodes, as previously reported (16). In NOD mice treated with Pam3CSK4 in vivo for 3 weeks, CD4+ diabetogenic T cell proliferation was significantly attenuated, indicating DC tolerance. Pooled results (right). A representative histogram (left). (B) Splenocytes prepared from NOD mice to which Pam3CSK4 was administered for 3 weeks, were further treated with Pam3CSK4 (black line) or PBS (gray filled histogram) in vitro for 1 day. The induction of costimulatory molecules on DCs by in vitro treatment with Pam3CSK4 that was determined by FACS analysis gated on CD11c+ cells was significantly lower compared to control NOD mice, suggesting DC tolerance. Fold changes of mean fluorescent intensities (MFI) (lower). (C) No significant difference in the expression of costimulatory molecules on splenic DCs between NOD mice that were treated with Pam3CSK4 in vivo (black line) and control NOD mice (gray line), suggests that in vivo Pam3CSK4 treatment for 3 weeks does not activate DCs. (***p<0.005; *p<0.05; ns, not significant) (Originally published in The Journal of Immunology. Kim D. H., J-C. Lee, S. Kim S, S. H. Oh, M.-K. Lee, K.-W. Kim, M.-S. Lee. 2011. Inhibition of autoimmune diabetes by TLR2 tolerance. J. Immunol.187: 5211-5220. Copyright © [2011] The American Association of Immunologists, Inc.) |
 | Figure 2Effect of in vivo treatment with Pam3CSK4 on diabetes transfer by sensitized diabetogenic T cells. The incidence of diabetes after adoptive transfer (AT) of splenocytes from diabetic NOD mice to irradiated nondiabetic recipient NOD mice was significantly reduced by treatment of the recipient NOD mice with Pam3CSK4 for 2 weeks before AT (p<0.005). (Originally published in The Journal of Immunology. Kim D. H., J-C. Lee, S. Kim S, S. H. Oh, M.-K. Lee, K.-W. Kim, M.-S. Lee. 2011. Inhibition of autoimmune diabetes by TLR2 tolerance. J. Immunol.187: 5211-5220. Copyright © [2011] The American Association of Immunologists, Inc.) |
T1D
TLR
DC
NOD
DPP4
Mφ
DAMP
APC
CRAMP
GLP-1
iPSc