Abbreviations
MHC
APC
CTLA-4
Fc
CD
MLR
ELISA
HRP
FITC
PBS
SDS-PAGE
TBST
RT-PCR
KDa
Kb
CPM
PVDF
Journal List > Immune Netw > v.13(1) > 1033382
MHC
APC
CTLA-4
Fc
CD
MLR
ELISA
HRP
FITC
PBS
SDS-PAGE
TBST
RT-PCR
KDa
Kb
CPM
PVDF
 | Figure 1The amino acid sequence of CTLA-4Ig. (A) Schematic diagram of mouse CTLA-4Ig amino acid sequence. Red, blue, green, and dark-red color represent extracellular domain of CTLA, hinge region, CH2, and CH3 region, respectively. (B) The electrophoresis of PCR product to change amino acids of end of the IgG3. After PCR reaction, 2µl of reaction mixture loaded into 1% agarose gel and followed by the electrophoresis. The size of product was estimated as 1.2 Kb. (C) In vitro transcription and translation of mutant and wild type CTLA-4Ig. pCDNA3.1 is a mock vector as negative control. Highly purified DNA was added to reaction mixture as described in the material and methods. The products of reaction were separated by SDS-PAGE and transferred to a PVDF membrane followed by incubation with streptavidin-HRP conjugated antibody and detection with using ECL Kit. Molecular weight of 45 KDa protein band was seen in both mutant and wild type, but not in mock vector. |
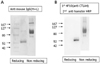 | Figure 2Western blot analysis of purified CTLA-4Ig-CTKC. Each 5µg of protein was added to the sample buffer with the β-ME (reducing condition) or without the β-ME (non reducing condition) and then separated by SDS-PAGE. (A) Fc Portion of the CTLA-4Ig-CTKC was detected by using an alkaline phosphatase (AP)-conjugated anti-mouse IgG antibody. Molecular weight of 52 KDa at reducing condition and 110 KDa at non-reducing condition were found. (B) The extracellular domain of CTLA-4 in the CTLA-4Ig-CTKC was detected by using anti-CTLA-4 monoclonal antibody, 4F10 and HRP-conjugated anti-hamster IgG. The same size of protein shown in (A) was detected. This figure was a representative from more than three independent experiments. |
 | Figure 3The binding assay of CTLA-4Ig and CTLA-4Ig-CTKC to the P815B7-1 cells. P815B7-1 cells (5×105) were stained with an isotype control antibody or with the FITC-conjugated anti-mouse CD80 as positive control (A). Equal numbers of P815B7-1 cells were incubated with 50, 1,000, and 20,000 ng/ml of CTLA-4Ig (B) or CTLA-4Ig-CTKC (C), respectively and then stained with the FITC-conjugated anti-mouse IgG. All of the data were analyzed by flow cytometry. The numbers inside the plot represent the percentage of CTLA-4Ig binding cells in P815B7-1 cells. This result is a representative from at least three independent experiments. |
 | Figure 4Inhibition of T cell proliferation of CTLA-4Ig-CTKC on the allogenic MLR. Splenocytes from C57BL/6 mice (5×105) were stimulated with equal number of irradiated splenocytes from BALB/c mice in the total volume of 0.2 ml and the different amounts of either CTLA-4Ig (A) or CTLA-4Ig-CTKC (B) were added at the same time. After 24, 48 and 72 hours incubation, 1µCi of 3H-thymidine was added to each sample for 18 hours. The amount of 3H-thymidine uptake by proliferating T cells in each well was estimated by counting per minute in the micro-beta counter. |
 | Figure 5Survival plots of mouse pancreatic islet allografts. The donor mouse (Balb/C) pancreatic islet cells were purified after collagenase digestion as described in material and methods. Diabetic recipient B6 mice received 500 IEQ of islets under left side kidney capsule. (A) Immediately after islet transplantation, B6 mice were injected with 50µg of CTLA-4Ig every other day for 14 days intraperitoneally (n=2). All of these mice kept their graft over 80 days. (B) Animals were treated with 50µg of CTLA-4Ig-CTKC (n=3) according to same injection procedure. |
MHC
APC
CTLA-4
Fc
CD
MLR
ELISA
HRP
FITC
PBS
SDS-PAGE
TBST
RT-PCR
KDa
Kb
CPM
PVDF