Abstract
Hypoxia has been shown to promote inflammation, including the release of proinflammatory cytokines, but it is poorly investigated how hypoxia directly affects inflammasome signaling pathways. To explore whether hypoxic stress modulates inflammasome activity, we examined the effect of cobalt chloride (CoCl2)-induced hypoxia on caspase-1 activation in primary mixed glial cultures of the neonatal mouse brain. Unexpectedly, hypoxia induced by oxygen-glucose deprivation or CoCl2 treatment failed to activate caspase-1 in microglial BV-2 cells and primary mixed glial cultures. Of particular interest, CoCl2-induced hypoxic condition considerably inhibited NLRP3-dependent caspase-1 activation in mixed glial cells, but not in bone marrow-derived macrophages. CoCl2-mediated inhibition of NLRP3 inflammasome activity was also observed in the isolated brain microglial cells, but CoCl2 did not affect poly dA:dT-triggered AIM2 inflammasome activity in mixed glial cells. Our results collectively demonstrate that CoCl2-induced hypoxia may negatively regulate NLRP3 inflammasome signaling in brain glial cells, but its physiological significance remains to be determined.
Inflammasome is assembled mainly in innate immune cells when the pattern-recognition receptor (PRR), such as Nod-like receptor (NLR) or absent in melanoma 2 (AIM2) senses a wide range of cytoplasmic abnormal signals derived from microbial infection or tissue injury (1,2). Assembled inflammasome then activates caspase-1, leading to the subsequent processing and secretion of interleukin-1-beta (IL-1β), which triggers proinflammatory responses. Initial activation of inflammasome signaling provides a primary defense against invading microbes, but many recent studies have reported that deregulated or sustained activation of inflammasome is associated with chronic inflammatory or metabolic diseases (3,4).
Previous investigations have also revealed that IL-1β is elevated in the central nervous system (CNS) under diverse pathological conditions and implicated in brain injury and chronic neurodegenerative diseases, including Alzheimer's diseases (5-7). Notably, amyloid-β, accumulated in senile plaques, has been shown to activate NLRP3 inflammasome signaling in microglial cells, resulting in the increased release of IL-1β (8). Furthermore, the deficiency of Nlrp3- or caspase-1 reduces the pathogenesis of Alzheimer's disease in a transgenic mouse model expressing a mutant amyloid precursor protein and a mutant presenilin 1 (9). These recent findings indicate that NLRP3 inflammasome is a crucial signaling axis responsible for inflammation-mediated neurotoxicity, leading to the neurodegenerative diseases.
Hypoxia normally occurs under many physiological conditions including ischemia and organ grafts (10). Tissue hypoxia promotes local inflammation as evidenced by accumulations of inflammatory cells and elevated levels of proinflammatory cytokines (11). In particular, the brain is highly susceptible to hypoxic or ischemic neuronal damage in case cerebral blood flow is temporarily blocked (12). Excitotoxicity and oxidative stress are mainly responsible for hypoxic or ischemic neuronal cell death (13), but inflammation, primarily by activated microglial cells, also plays a crucial role in exacerbating hypoxic brain injury (14). Indeed, previous reports have demonstrated that caspase-1 is critical for neuronal cell death under hypoxic or ischemic stress (15,16). However, it is still unclear whether hypoxia could stimulate or potentiate assembly of the inflammasome complex and subsequent activation of caspase-1, especially in brain glial cells. In this study, we thus examined the effect of hypoxia on inflammasome activation in mixed glial cells from the neonatal mouse brain.
Mouse primary mixed glial cells were isolated and cultured as described previously (12). Briefly, the whole brain from pups on the first postnatal day was isolated and the meninges were removed in chilled Hanks' balanced salt solution. The brain was then dissociated in DMEM/F-12 medium containing trypsin-EDTA and incubated in 5% CO2 for 12 min. The brain homogenate was centrifuged and filtered by using a cell strainer (100µm). Dissociated cells were washed and plated onto a 100-mm culture dish, and the medium was replaced every 3 days for 2~3 weeks. To isolate microglial cells, the above brain-mixed cultures were agitated for 8 h, and the liberated cell fraction was used for microglial cells. Mouse immortalized bone marrow-derived macrophages were prepared as described previously (17). To induce oxygen-glucose deprivation (OGD), culture medium was replaced with glucose-free DMEM, and the cells were placed in a humidified 37℃ incubator containing a mixture of 95% N2 and 5% CO2 for the indicated times.
Anti-human/mouse caspase-1 antibody was obtained from Santa Cruz (Santa Cruz, CA, USA) and kindly gifted from Dr. Emad Alnemri (Thomas Jefferson University). Anti-human/mouse IL-1β antibody was purchased from Cell Signaling Technology (Beverly, MA, USA) and R&D (Minneapolis, MN, USA). All the other antibodies were obtained from Cell Signaling Technology (PARP), Alexis (San Diego, CA, NLRP3), Abcam (Cambridge, MA, USA, HIF-1α) and eBioscience (San Diego, CA, CD11b-PE and F4/80-APC). LPS, CoCl2, nigericin, ATP, and poly dA:dT were obtained from Sigma (St Louis, MO, USA), and z-VAD-fluoromethylketone was from Bachem (Torrance, CA, USA). All the culture media and supplements were purchased from Invitrogen (Grand Island, NY, USA).
Cells were lysed in a buffer containing 20 mM HEPES (pH 7.5), 0.5% NP-40, 50 mM KCl, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, and protease inhibitors. Cell lysates were fractionated on SDS-PAGE, transferred onto PVDF membranes (BioRad, Hercules, CA, USA), and then Western-blotted using appropriate antibodies as indicated. Cell culture supernatants were precipitated by methanol/chloroform as described previously (17) and immunoblotted with appropriate antibodies.
To quantitate microglial cell population, cells were stained with anti-CD11b-PE and anti-F4/80-APC antibodies, and analyzed by flow cytometry. To detect mitochondrial ROS, cells were stained with MitoSox (Invitrogen) according to the manufacturer's protocol and analyzed by flow cytometry.
To test whether hypoxia or ischemia could trigger inflammasome signaling in brain microglial cells, we first observed caspase-1 processing in microglial BV-2 cells after oxygen-glucose deprivation (OGD), which induces ischemia-like injury. Extracellular release of processed caspase-1, a crucial readout of inflammasome activation, was not detected even by 12-h duration of OGD, while the secretion of processed IL-1β was observed in the cultural supernatant of BV-2 cells under OGD conditions longer than 6 h (Fig. 1A). This result suggests that OGD could induce IL-1β release in a caspase-1/inflammsome-independent manner.
Previous reports demonstrated that OGD causes brain cell death of not only neuron but also microglia (18,19), suggesting the possibility that the above OGD-mediated processing and secretion of IL-1β is due to cell death, but not to caspase-1/inflammasome. Indeed, longer duration of OGD than 8 h resulted in apoptotic cell death of BV-2 cells as determined by the cleavage of PARP (Fig. 1A, lower panel). We thus examined whether the OGD-mediated IL-1β release is caspase-dependent or caspase-independent using a pan-caspase inhibitor zVAD. As described in the above experiment, only processed IL-1β, but not caspase-1, was detected in the supernatants of BV-2 cells upon OGD treatment (Fig. 1B). However, the caspase inhibitor zVAD did not reduce the processing and release of IL-1β (Fig. 1B), indicating that IL-1β may be released by OGD in a caspase-independent manner.
The released IL-1β by OGD seems rather fragmented than processed into active IL-1β regardless of caspase activity (Fig. 1B). All these observations suggest that ischemia may not induce assembly of inflammasome and activation of caspase-1, but trigger nonspecific fragmentation and release of IL-1β, probably by cell death. Similar to the ischemia-mimicking OGD treatment, CoCl2-induced hypoxia did not promote caspase-1 activation and IL-1β secretion in BV-2 cells, verifying that hypoxia is not implicated in the activation of inflammasome in microglial cells (Fig. 1C).
Then, we examined the effect of CoCl2 on inflammasome signaling in the primary mixed glial cultures. As observed in BV-2 cells, CoCl2 did not trigger caspase-1 activation in the LPS-primed mixed glial cells, whereas nigericin, a well-known NLRP3 stimulator, clearly induced the processing of caspase-1 (Fig. 2A). CoCl2 treatment remarkably increased the monomeric or oligomeric level of HIF-1α, indicating that CoCl2 induces hypoxic condition in brain mixed glial cultures. In contrast to the OGD treatment, no IL-1β was released by CoCl2 treatment in LPS-primed mixed glial cells (Fig. 2A), demonstrating that hypoxia is not likely to induce inflammasome activation. To verify these observations in other cell types than brain mixed glial cells, we treated LPS-primed mouse bone marrow macrophages and PMA-differentiated THP-1 macrophages with CoCl2. As observed in mixed glial cultures, CoCl2-induced hypoxic conditions failed to activate caspase-1 in both macrophages, but NLRP3 stimulators, such as nigericin and LPS, triggered a robust activation of caspase-1, (Fig. 2B and C). All the above results indicate that hypoxia may not be a critical stimulus to the activation of inflammasome signaling.
Next, we examined whether CoCl2-induced hypoxic conditions facilitate or potentiate inflammasome signaling in primary mixed glial cells, as hypoxia has been implicated in the development of inflammation (10). Interestingly, CoCl2 did not potentiate, but rather inhibit ATP- or nigericin-induced NLRP3 inflammasome activation in LPS-primed brain mixed glial cells, as extracellular release of caspase-1 and IL-1β in their mature forms was attenuated in the presence of CoCl2 (Fig. 3A). On the contrary, CoCl2 had no effect on AIM2 inflammasome activity in response to synthetic double-stranded DNA, poly dA:dT (17) (Fig. 3B). This observation demonstrates that CoCl2 may unexpectedly inhibit NLRP3-mediated caspase-1 activation in brain mixed glial cells.
To lineate the regulative role of CoCl2 on NLRP3 inflammasome in bone marrow-derived macrophages, we determined active caspase-1 and IL-1β in the cultural supernatants of LPS-primed bone marrow macrophages after stimulation with ATP or nigericin. Unlike mixed glial cultures, NLRP3 inflammasome activity was not affected by CoCl2 treatment in bone marrow macrophages (Fig. 3C), indicating that CoCl2-induced hypoxia may play a unique inhibitory role on NLRP3 inflammasome of brain mixed glial cells.
Then, we determined the effect of CoCl2 at a various treatment time before ATP stimulation to provide a molecular insight into the regulation of NLRP3 inflammasome by CoCl2. As shown in Fig. 4A, longer treatment of CoCl2 than 4 h reduced ATP-mediated caspase-1 activation and IL-1β release, but 1-h treatment of CoCl2 was not sufficient to attenuate NLRP3 inflammasome activation. This result suggests that CoCl2 might not directly interfere with the interaction of NLRP3 and ASC or with active assembly of NLRP3 inflammasome, because NLRP3 inflammasome is activated only after ATP stimulation. We can infer that a new protein synthesis might be required for the negative regulation of NLRP3 inflammasome by CoCl2. The effect of CoCl2 was also examined with various LPS priming times. CoCl2 effectively attenuated NLRP3-dependent caspase-1 activation and IL-1β release in mixed glial cultures regardless of LPS-priming time (Fig. 4B).
In the central nervous system, microglia is the primary cell type responsible for recognizing abnormal molecular pattern, such as ATP, from tissue injury and triggering inflammatory responses (20). According to the previous report, microglia represents only 10% of the mixed glial cultures from the neonatal mouse brain (12). To exclude the possibility that the above finding was resulted from other cell types than microglia, we isolated microglia from mixed glial cultures. Similar to the previous report, the population of microglia was 6.7% among the mixed glial cultures, as determined by the cell populations expressing both CD11b and F4/80 (Fig. 5A). In contrast, microglial cells were enriched by 81.6% after isolation by the shaking method (Fig. 5A).
Next, we examined NLRP3 inflammasome activity in both mixed glial culture and isolated microglial cell. ATP-mediated caspase-1 activation and IL-1β release were similarly decreased by CoCl2 cotreatment not only in mixed glial cultures but also in microglial cells (Fig. 5B). This data indicates that CoCl2-induced hypoxia may negatively regulate NLRP3 inflammasome activity in brain microglial cells.
Although it is still unclear how NLRP3 inflammasome is activated upon a variety of stimuli including microbial toxins and crystalline substances, mitochondrial reactive oxygen species (ROS) has recently been suggested as a critical mediator facilitating the assembly of NLRP3 inflammasome (21). We thus examined whether mitochondrial ROS is important for ATP-induced NLRP3 inflammasome activation in brain mixed glial cells and whether CoCl2-mediated inhibition of NLRP3 signaling is dependent on mitochondrial ROS levels. In bone marrow-derived macrophages, ATP treatment caused a significant production of mitochondrial ROS, albeit to a lesser extent than rotenone which specifically damages the electron transport chain complex I leading to the generation of mitochondrial ROS (Fig. 6A). Contrary to bone marrow-derived macrophages, ATP failed to produce mitochondrial ROS in LPS-primed brain mixed glial cells (Fig. 6B), suggesting that mitochondrial ROS may be dispensable for ATP-mediated caspase-1 activation in mixed glial cells. Furthermore, CoCl2 had no effect on the generation of mitochondrial ROS (Fig. 6B). Taken together, CoCl2-mediated inhibition of NLRP3 inflammasome activity in brain mixed glial cells seems independent of mitochondrial ROS production.
Inflammation is primarily a defense system against microbial infection or tissue injury, but excessive inflammation can cause host cellular or tissue damage. The caspase-1/inflammasome signaling pathway is activated upon a wide range of pathogen- or damage-associated molecular patterns and responsible for the secretion of proinflammatory cytokines IL-1β and IL-18 (2). Emerging evidence demonstrates that deregulated inflammasome signaling is implicated not only in inflammatory disorders but also in metabolic diseases, including type II diabetes, obesity, and atherosclerosis (3,4,22). It has thus been of interest to elucidate molecular mechanisms by which inflammasome signaling is regulated in response to diverse stimuli.
Brain is a highly susceptible organ for inflammation-mediated injury to neurons especially under hypoxic conditions after cerebral ischemia or stroke (7). It has already been reported that hypoxia induces cerebral damage via triggering IL-1β-mediated toxicity to neurons and the deficiency or inactivation of caspase-1 confers neuroprotection against hypoxic neuronal damage (7,15,23). In this regard, we expected that hypoxic conditions could trigger or at least potentiate the inflammasome/caspase-1 signaling pathway. However, our results demonstrated that hypoxia did not promote caspase-1 activation but inhibited the NLRP3-mediated caspase-1 signaling pathway.
So far, it is not well investigated whether hypoxic stimuli directly regulate the inflammasome signaling pathway. On the basis of previous results, hypoxic or ischemic injury definitively induces necrotic cell death in the brain, and released danger-associated molecular patterns from necrotic cells might activate the NLRP3 inflammasome signaling pathway, leading to the initiation of inflammatory responses (23). Therefore, inhibition of caspase-1-mediated signaling could diminish neurotoxicity from hypoxia-induced IL-1β. Nevertheless, this study proposed that hypoxia itself did not directly activate the caspase-1/inflammasome signaling pathway in brain mixed glial cells containing microglia but rather provided a negative regulation of NLRP3 inflammasome. At present, it is not well understood why hypoxia inhibits the inflammasome signaling in brain mixed glial cells. Therefore, it needs to be clarified by further investigation to provide a molecular insight explaining this phenomenon.
Figures and Tables
Figure 1
Hypoxia does not activate caspase-1 in BV-2 cells. Cultural supernatants or lysates were immunoblotted as indicated. (A) BV-2 cells were resuspended in glucose-free media and incubated in hypoxic chamber for the indicated times. (B) BV-2 cells were treated with OGD as described in (A) for 5 h in the presence or absence of zVAD (20µM). (C) BV-2 cells were treated with LPS (0.25µg/ml) for 3 h, followed by treatment with CoCl2 (100µM) in the presence or absence of zVAD (20 µM) for 15 h.
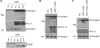
Figure 2
CoCl2-induced hypoxia does not induce caspase-1 activation in primary mixed glial cultures. (A) Immunoblots for the indicated antibodies in cultural supernatants or lysates of brain mixed glial cells treated with LPS (0.25µg/ml) with or without CoCl2 (100µM) for the indicated times or LPS followed with nigericin (5 mM, final 45 min) as indicated. (B) Mouse bone marrow-derived macrophages were treated with LPS (0.25µg/ml) for 3 h, followed by treatment with CoCl2 (100µM) or nigericin (5µM, 45 min) as indicated. (C) PMA-differentiated THP-1 cells were treated with CoCl2 (100µM) or LPS (0.5µ g/ml, 6 h). Soluble lysates and supernatants were immunoblotted as indicated antibodies. Note that the processed caspase-1 p10 or p20 band is detected depending on the p10- or p20-specific anti-caspase-1 antibody.
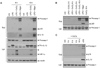
Figure 3
CoCl2-induced hypoxia attenuates NLRP3-dependent caspase-1 activation in primary mixed glial cultures. (A, B) Immunoblots for the indicated antibodies in cultural supernatants or lysates of brain mixed glial cells treated with LPS (0.25µg/ml) with or without CoCl2 (100µM) for 7 h, followed by treatment with ATP (5 mM, 45 min) or nigericin (5µM, 45 min), or transfected with poly dA:dT (1µg, 6 h) with or without CoCl2 (100µM). (C) Immunoblots for the indicated antibodies in cultural supernatants or lysates of bone marrow-derived macrophages treated with LPS (0.25µg/ml) with or without CoCl2 (100µM) for 7 h, followed by treatment with ATP (5 mM, 45 min) or nigericin (5µM, 45 min).

Figure 4
Time course effect of CoCl2 on the NLRP3 inflammasome activity. (A) Immunoblots for the indicated antibodies in cultural supernatants or lysates of brain mixed glial cells treated with LPS (0.25 µg/ml, 7 h) with or without CoCl2 (100µM, indicated times), followed by treatment with ATP (5 mM, 45 min). Note that the CoCl2 of the sixth lane was cotreated with ATP. (B) Immunoblots for the indicated antibodies in cultural supernatants or lysates of brain mixed glial cells treated with LPS (0.25µ g/ml) with or without CoCl2 (100µM) for the indicated times, followed by treatment with ATP (5 mM, 45 min).

Figure 5
CoCl2-induced hypoxia reduces NLRP3-dependent caspase-1 activation in primary microglial cells. (A) Flow cytometric analysis of mouse brain mixed glial cells (upper) and isolated microglial cells (lower) using anti-CD11b-PE and anti-F4/80 antigen-APC antibody. (B) Immunoblots for the indicated antibodies in cultural supernatants of brain mixed glial cells or isolated microglial cells treated with LPS (0.25µg/ml) with or without CoCl2 (100µM) for 7 h, followed by treatment with ATP (5 mM, 45 min).
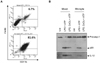
Figure 6
Mitochondrial ROS is not critical for NLRP3 inflammasome activation in primary mixed glial cells. (A) Flow cytometric analysis of mouse bone marrow-derived macrophages treated with rotenone (10µM, 1 h) or ATP (3mM, 30 min) and stained with MitoSox. (B) Flow cytometric analysis of brain mixed glial cells treated with rotenone (10µM, 2 h) or LPS (0.25µg/ml) with or without CoCl2 (100 µM) for the indicated times, followed treatment with ATP (5 mM, 40 min) and stained with MitoSox.

ACKNOWLEDGEMENTS
This work was supported in part by Basic Science Research Program through NRF funded by MEST (2010-10849), and by National R&D program for Cancer Control, Ministry for Health, Welfare and Family affairs, Rep of Korea (1020190), and BK 21 Project for Medical Science.
References
2. Hong S, Park S, Yu JW. Pyrin domain (PYD)-containing inflammasome in innate immunity. J Bacteriol Virol. 2011; 41:133–146.

3. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012; 481:278–286.

4. Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012; 13:352–357.

5. Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008; 5:7.

6. Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A. 2010; 107:13046–13050.
7. Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends Pharmacol Sci. 2011; 32:617–622.

8. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008; 9:857–865.

9. Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013; 493:674–678.

11. Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009; 10:195–202.

12. Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-Inflammasome Activating DAMPs Stimulate an Inflammatory Response in Glia in the Absence of Priming Which Contributes to Brain Inflammation after Injury. Front Immunol. 2012; 3:288.

13. Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002; 35:67–86.

14. Johnson DR, O'Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007; 27:1161–1166.

15. Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A. 2003; 100:16012–16017.

16. Bossenmeyer-Pourié C, Koziel V, Daval JL. Involvement of caspase-1 proteases in hypoxic brain injury. effects of their inhibitors in developing neurons. Neuroscience. 2000; 95:1157–1165.

17. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009; 458:509–513.

18. Eyo UB, Miner SA, Ahlers KE, Wu LJ, Dailey ME. P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation. Neuropharmacology. 2013; 73C:311–319.

19. Almeida A, Delgado-Esteban M, Bolaños JP, Medina JM. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem. 2002; 81:207–217.

20. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011; 11:775–787.

21. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011; 469:221–225.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download