Abstract
Graft-versus-host disease (GVHD) is a common complication of allogeneic stem cell transplantation (allo-SCT). However, a similar syndrome has been reported in autologous stem cell transplantation (ASCT) as well. The target organs of GVHD in ASCT are the skin, liver and gastrointestinal (GI) tract, which are consistent with those in allo-SCT. Histologic findings from the skin and the mucosa of the GI tract also show similar features. Here we describe a case of autologous GVHD involving the skin of a patient who underwent ASCT for multiple myeloma. In this patient, the response to a total prednisone dose of 0.5 mg/kg/day was unsatisfactory, and the patient required more intensive and prolonged immunosuppressive therapy with slow tapering.
Graft-versus-host disease (GVHD) is a common complication of allogeneic stem cell transplantation (allo-SCT). GVHD that occurs after allo-SCT is attributable to the recognition of donor T cells or natural killer cells by recipient alloantigens. However, a similar syndrome has been reported in autologous stem cell transplantation (ASCT), without induction of an alloresponse, as a form of 'auto-aggression' syndrome or autologous GVHD (auto-GVHD) (1). Although there is no genetic disparity in GVHD development among patients undergoing ASCT, auto-GVHD has been reported to arise either spontaneously (2-5) or in patients receiving immunologic modulation (6,7). Auto-GVHD is generally self-limited and often requires no treatment (8-10). In contrast, several case reports have described patients presenting with severe and ultimately fatal spontaneous auto-GVHD (2-4). In particular, individuals treated with ASCT for multiple myeloma (MM) may develop potentially life-threatening syndromes which are pathologically identical to allogeneic GVHD. Fidler et al. (2) described a case of auto-GVHD with skin and GI manifestations in a patient with MM, who ultimately deteriorated and expired due to auto-GVHD. Another case series by Drobyski et al. (3) described 5 patients who developed clinical syndrome consistent with auto-GVHD. In their case series, 4 of the 5 patients ultimately expired due to complications directly attributed to steroid-refractory auto-GVHD.
Here we describe a case of auto-GVHD involving the skin after ASCT for MM. In this patient, the response to a total prednisone dose of 0.5 mg/kg/day was unsatisfactory, and the patient required more intensive immunosuppressive therapy for several months with slow tapering.
A 59-year-old man was diagnosed with kappa-light chain MM. Protein electrophoresis and assays revealed elevated kappa-light chain levels. He had a serum albumin level of 3.0 g/dl, a β2 microglobulin level of 12.6 mg/L, and renal insufficiency. Bone marrow examination revealed 39% atypical plasma cells with complex karyotypes including t(11;14), and plain radiographs showed multiple compression fractures in the thoracic vertebrae. The patient received induction chemotherapy with bortezomib, melphalan, and prednisone; he achieved a partial response after 2 courses of this regimen. He was then treated with a high dose of melphalan (200 mg/m2), followed by stem cell support in a cell dose of 4.65×106 CD34+ cells/kg.
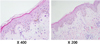
On day 19 post-transplantation, the patient presented with fever and diffuse, erythematous, and maculopapular rashes covering 54% of his body surface area, including the trunk and extremities. Laboratory examination revealed mild anemia and a normal leukocyte count/differentiation. The C-reactive protein level was increased to 6.38 mg/dl (reference range, 0~0.3 mg/dl). However, an extensive workup for infectious etiologies was negative, and empiric antibiotic therapy proved to be ineffective. A skin biopsy taken from the trunk revealed exocytosis with basal vacuolar degeneration and sparse inflammatory infiltration in the dermis, being consistent with findings of auto-GVHD (Fig. 1). Periodic Acid Schiff (PAS), Gomori Methenamine Silver (GMS), and Ziehl-Neelson stainings of tissue sections were all negative, as were tissue cultures for bacteria and fungi. The skin rashes progressed, becoming intensely erythematous with desquamation. To treat such auto-GVHD symptoms, the patient was started on prednisone (0.5 mg/kg/day). The skin rashes began to resolve, but tapering of the steroid worsened his skin rashes, subsequently disturbing reduction in steroid doses for approximately 3 months.
On day 112 post-transplantation, the patient developed dyspnea with fever. He was diagnosed with Peumocystis jeroveci peumonia (PJP), and his conditions improved after sulfamethoxazole/trimethoprim (bactrim) treatment along with methylprednisolone (1 mg/kg) IV daily. It was noted that his skin rashes improved while the steroid doses were increased to treat hypoxia due to PJP. However, tapering of the steroid led to an aggravation of skin lesions with generalized erythroderma and desquamation. Eventually, the patient was re-started on methylprednisolone (1 mg/kg) IV daily. Currently, his skin rashes have improved on slow prednisone tapering (Fig. 2).
GVHD is a common complication of allo-SCT which is induced by donor T-cell recognition of recipient alloantigens. However, a few studies have reported syndromes that are clinically and histologically similar to allogeneic GVHD in patients who have undergone ASCT. The incidence of Auto-GVHD has been reported to be approximately 5~20% (4,8,9). Auto-GVHD can involve the skin, intestinal tract, and liver which are also target organs of allogeneic GVHD (3,11,12). Skin involvement has been reported to be the most frequent in auto-GVHD (4,8). In contrast to allogeneic GVHD, auto-GVHD has a milder course, and most patients resolve spontaneously or respond to corticosteroids (8-10). However, it has been reported that auto-GVHD is refractory to steroid therapy and ultimately fatal in some patients (2-4). In our case, auto-GVHD involving the skin did not respond to a total prednisone dose of 0.5 mg/kg/day. To make matters worse, the patient developed PJP while receiving systemic corticosteroid therapy without bactrim prophylaxis. Notably, the patient's skin lesion improved after the steroid dose was increased to treathypoxia due to PJP. Our case report indicates that auto-GVHD may require prompt and intensive immunosuppressive therapy and that bactrim prophylaxis may also be needed.
The incidence of auto-GVHD is higher in patients undergoing ASCT for MM than in those with acute myelogenous leukemia, non-Hodgkin lymphoma, or Hodgkin disease. In particular, the risk for auto-GVHD is higher in patients undergoing tandem transplantation (12%) for MM than in those undergoing a single session of transplantation (0.9%). Auto-GVHD after tandem transplantation is likely to be a more severe form (3,4,9,13). One possible explanation for this is that alteration of immune function which causes auto-GVHD may occur as a result of the disease process of MM or the treatment regimen. Fidler et al. (2) have suggested that the failure of self-tolerance and development of auto-GVHD may be caused by bortezomib therapy which induces apoptosis. Deficient clearance of apoptotic cells may lead to presentation of auto-antigens to cytotoxic T-cells. Lazarus et al. (13) evaluated the expression of surface markers in infused hematopoietic progenitor grafts of patients who developed auto-GVHD and suggested that exposure to immunomodulating therapies for induction treatment affects not only CD34+ cells but also T cells or relevant T-cell subpopulations, which in turn can mediate GVHD. Our patient also received bortezomib therapy prior to ASCT as an induction regimen.
In summary, when skin lesions occur after ASCT, physicians always keep in mind auto-GVHD in the differential diagnosis of the lesions. Patients with auto-GVHD can be successfully managed with prompt and intensive immunosuppressive therapy along with prophylactic treatment for PJP.
Figures and Tables
References
1. Kline J, Subbiah S, Lazarus HM, van Besien K. Autologous graft-versus-host disease: harnessing anti-tumor immunity through impaired self-tolerance. Bone Marrow Transplant. 2008. 41:505–513.

2. Fidler C, Klumpp T, Mangan K, Martin M, Sharma M, Emmons R, Lu M, Kropf P. Spontaneous graft versus host disease occurring in a patient with multiple myeloma after autologous stem cell transplant. Am J Hematol. 2012. 87:219–221.

3. Drobyski WR, Hari P, Keever-Taylor C, Komorowski R, Grossman W. Severe autologous GVHD after hematopoietic progenitor cell transplantation for multiple myeloma. Bone Marrow Transplant. 2009. 43:169–177.

4. Goddard DS, Ruben BS, Mathes ED, Nixon M, Wolf J, Fox LP. A case of severe cutaneous, GI and liver GVHD in a patient with multiple myeloma, status-post-second auto-SCT. Bone Marrow Transplant. 2010. 45:409–411.

5. Krishna SG, Barlogie B, Lamps LW, Krishna K, Aduli F, Anaissie E. Recurrent spontaneous gastrointestinal graft-versus-host disease in autologous hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2010. 10:E17–E21.

6. Baron F, Gothot A, Salmon JP, Hermanne JP, Pierard GE, Fillet G, Beguin Y. Clinical course and predictive factors for cyclosporin-induced autologous graftversus-host disease after autologous haematopoietic stem cell transplantation. Br J Haematol. 2000. 111:745–753.

7. Jones RJ, Vogelsang GB, Hess AD, Mann RB, Geller RB, Piantadosi S, Santos GW. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989. 1:754–757.

8. Hood AF, Vogelsang GB, Black LP, Farmer ER, Santos GW. Acute graft-vs-host disease. Development following autologous and syngeneic bone marrow transplantation. Arch Dermatol. 1987. 123:745–750.

9. Holmberg L, Kikuchi K, Gooley TA, Adams KM, Hockenbery DM, Flowers ME, Schoch HG, Bensinger W, McDonald GB. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2006. 12:226–234.

10. Saunders MD, Shulman HM, Murakami CS, Chauncey TR, Bensinger WI, McDonald GB. Bile duct apoptosis and cholestasis resembling acute graft-versus-host disease after autologous hematopoietic cell transplantation. Am J Surg Pathol. 2000. 24:1004–1008.

11. Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Mod Pathol. 2011. 24:117–125.

12. Nellen RG, van Marion AM, Frank J, Poblete-Gutiérrez P, Steijlen PM. Eruption of lymphocyte recovery or autologous graft-versus-host disease? Int J Dermatol. 2008. 47:Suppl 1. 32–34.

13. Lazarus HM, Sommers SR, Arfons LM, Fu P, Ataergin SA, Kaye NM, Liu F, Kindwall-Keller TL, Cooper BW, Laughlin MJ, Creger RJ, Barr PM, Gerson SL, Kaplan D. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: flow cytometric analysis of hematopoietic progenitor cell grafts. Biol Blood Marrow Transplant. 2011. 17:970–978.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download