INTRODUCTION
MATERIALS AND METHODS
Animals
Cell culture and reagents
Cell viability and proliferation assays
Isotype-specific ELISA
Statistical analysis
RESULTS AND DISCUSSION
CpG induces mouse B cell growth and Igs production in a dose-dependent manner
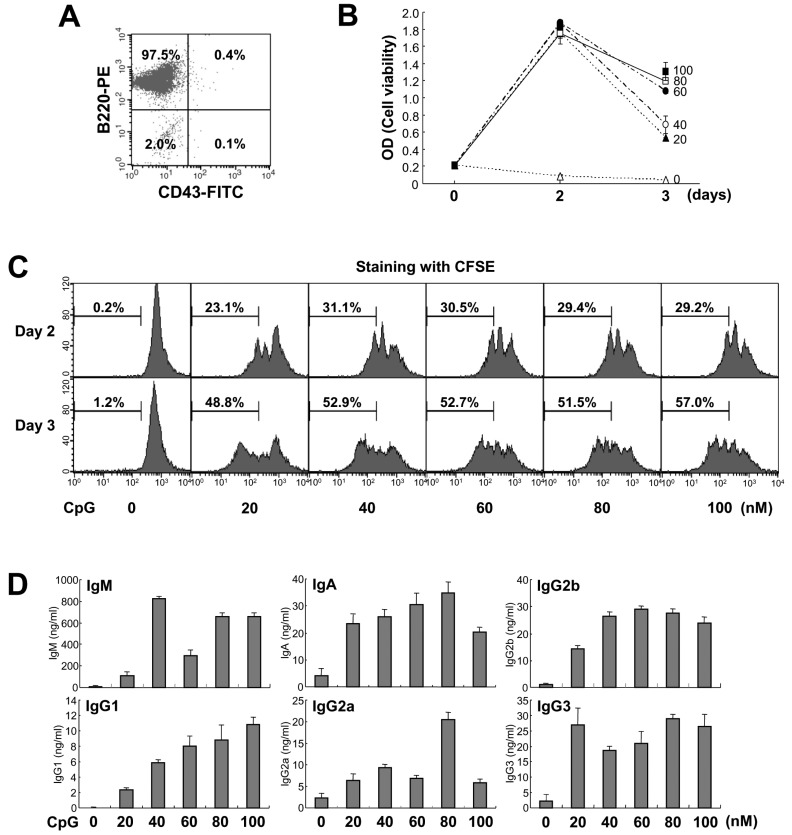 | Figure 1Effect of CpG on mouse B cell growth and Igs production. (A) Purified mouse spleen resting B cell population (CD43-B220+) was measured by flow cytometric analysis. Data shown are representative of all the experiments carried out in this study. (B) Resting B cells were stimulated with the indicated doses of CpG (0 nM, open triangle; 20 nM, closed triangle; 40 nM, open circle; 60 nM, closed circle; 80 nM, open square; 100 nM, closed square). After 2 and 3 days of culture, cell viability was measured by EZ-Cytox cell viability assay. Data are averages of duplicate samples with ranges (bars). (C) Culture conditions were the same as in (B). After 2 and 3 days of culture, cell proliferation was measured by the dilution of CFSE. The percentage indicates cells (%) with low level of CFSE intensity. (D) Culture conditions were the same as in (B). After 7 days of culture, supernatants were harvested and the levels of Igs production were determined by isotype-specific ELISA. Data represent means±SEM of triplicate samples. |
CpG is more potent than LPS in cell proliferation but lesser in Igs production
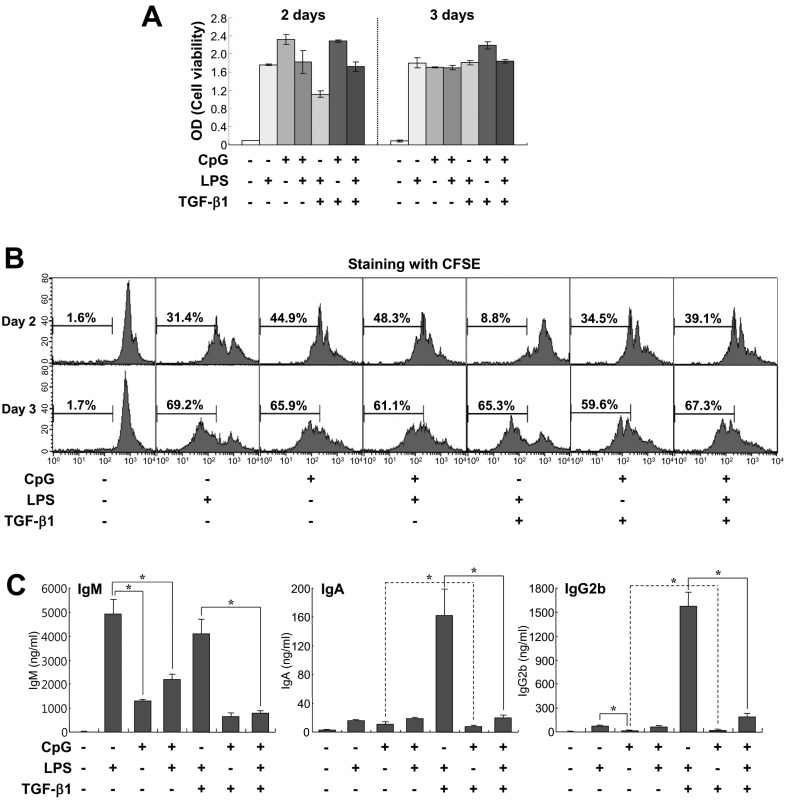 | Figure 2Comparison of effects of CpG and LPS on B cell growth and Igs production. Mouse spleen resting B cells were stimulated with CpG (100 nM), LPS (12.5µg/ml), and TGF-β1 (0.2 ng/ml). (A) After 2 and 3 days of culture, cell viability was measured by EZ-Cytox cell viability assay. Data are averages of duplicate samples with ranges (bars). (B) After 2 and 3 days of culture, cell proliferation was measured by the dilution of CFSE. (C) After 7 days of culture, supernatants were harvested and the levels of Igs production were determined by isotype-specific ELISA. Data represent means±SEM of triplicate samples. *p<0.05. |
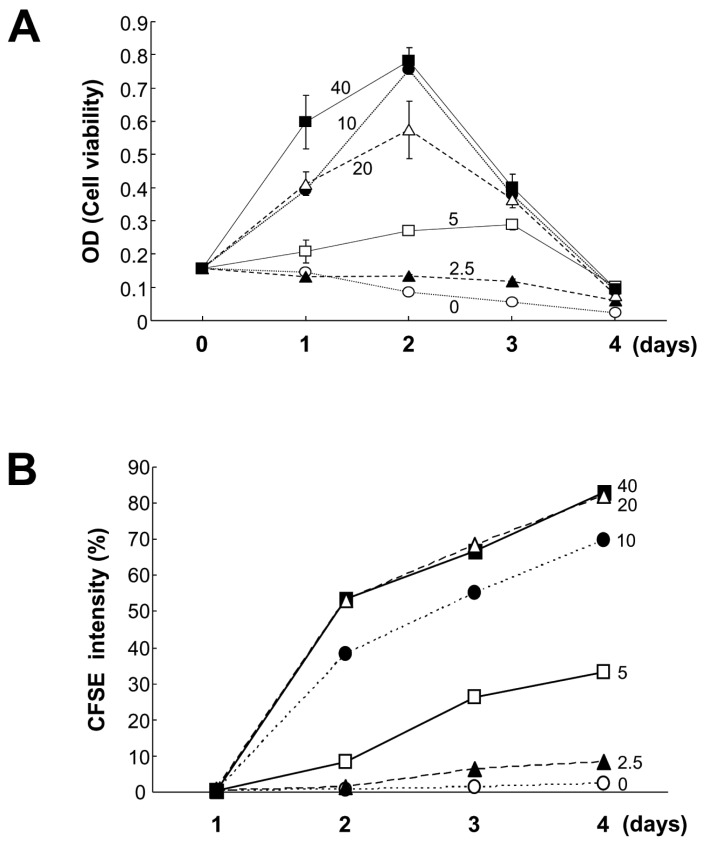 | Figure 3Effects of lower doses of CpG on B cell viability and proliferation. Mouse spleen resting B cells were stimulated with the indicated doses of CpG (0 nM, open circles; 2.5 nM, closed triangles; 5 nM, open squares; 10 nM, closed circles; 20 nM, open triangles; 40 nM, closed squares). After 1, 2, 3, and 4 days of culture, cell viability was measured by EZ-Cytox cell viability assay (A), and cell proliferation was measured by the dilution of CFSE (B). CFSE intensity (%) of panel B represents the percentage of cells with a low level of CFSE intensity. Data are averages of duplicate samples with ranges (bars). |
Effect of low dose CpG on TGF-β1- and RA-induced Igs production
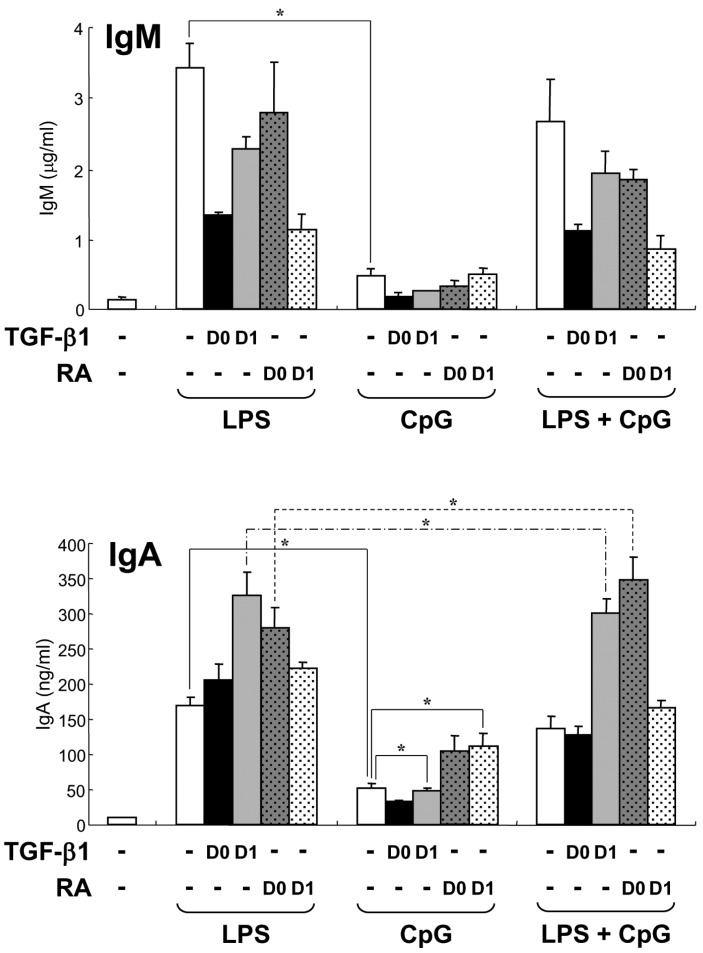 | Figure 4Effect of low dose CpG on TGF-β1- and RA-induced Igs production. Mouse spleen resting B cells were stimulated with CpG (10 nM) and LPS (12.5µg/ml). TGF-β1 (0.2 ng/ml) and retinoic acid (RA, 20 nM) were added on day 0 (D0) or day 1 (D1). After 7 days of culture, supernatants were harvested and the levels of IgM and IgA production were determined by isotype-specific ELISA. Data represent means±SEM of triplicate samples. *p<0.05. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download