INTRODUCTION
In immunology, an immunological synapse (IS) is the interface between a T cell and an antigen-presenting cell (APC) (1). It was first discovered and recognized in 1995 by Abraham Kupfer at the National Jewish Medical and Research Center in Denver and the term was coined by Michael Dustin at New York University who studied it in further detail (2). Since then, the clustering of receptors and intracellular proteins at the IS was visualized and the three-dimensional contact domains in T cell-APC interface, containing distinct surface molecules, were named 'supramolecular activation clusters' (SMACs) (3).
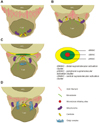
Micro- or nano-scale imaging of fixed T cell-APC interfaces or T cell-lipid bilayers coated with peptide-major histocompatibility complex (MHC) and intracellular adhesion molecule-1 (ICAM-1) reveals that immunological synapses in the mature state have a striking radial symmetry at the contact site and can be divided into three parts via separate distribution of some molecules (3). A large cluster of T cell receptor (TCR)-MHCp (MHC-presenting peptide) interactions is observed at the centre of the contact site, called the central-SMAC (c-SMAC), surrounded by a ring of lymphocyte function-associated antigen 1 (LFA-1) and ICAM-1 adhesion molecules (peripheral-SMAC, p-SMAC). Outside the p-SMAC is an actin and CD45-rich ring (CD stands for cluster of differentiation), called the distal-SMAC (d-SMAC). Once formed, the immunological synapse leads to sustained TCR-mediated signalling, stabilizes adhesion, and controls exocytosis and endocytosis, which allows directed cytokine or granule release and receptor internalization (4,5).
Dynamic rearrangements of actin cytoskeleton are necessary for the various effective functions of immune cells, as they provide powerful mechanical force like an inside cellular engine (6), by which immune cells can migrate, polarize, and exert effector functions. In many cases, actin regulation is overlapped between different processes in the cell. For example, both formation of lamellipodia during rapid migration and actin reorganization at the contact zones of pathogens and host cells are similar processes in terms of cytoskeletal rearrangements, as common actin regulators are recruited at the site of lamellipodium and interface of T cell-pathogen interaction (7,8). The regulation of cytoskeleton is extensively explored, but lots of aspects, e.g., how cells manage to effectively exploit actin network in different situations still need to be determined. This review briefly discusses recent works of actin cytoskeletal regulation at the IS.
Formation of IS is dynamic
The mobility is an in-built feature of immune cells. Specifically, it is crucial for cellular immunity during immune cell migration and interaction with each other in order to accomplish their function. Recent conception of synapse and kinapse formation between T cells and APCs reveals that interaction of T cell with APC starts by scanning. Transient adhesive junction - kinapse allows immune cells to facilitate signal integration while moving over the surface of APCs (9). IS is stabilized when cells halt their movement after recognizing less than 10 specific MHC-peptide complexes on the surface of the APCs (10). Then naïve T cells maintain long contacts with APCs in order to differentiate into effector T cells. Naïve T cell's commitment to proliferation requires sustaining of IS up to 20 h, whereas effector T cells only need 1 h of signalling for the commitment. On the contrary, prolonged antigenic stimulation may lead to the elimination of T cells (11,12).
Migration, transient adhesion and long stable interaction between immune cells demand accurate and fast rearrangements of actin cytoskeleton. The importance of actin cytoskeleton for IS was extensively explored in T cell biology. Interestingly, inhibition of actin function in B cells did not prevent movement of surface proteins at the IS zone (13,14), whereas interfering of actin or myosin function in T cell side prevented the trafficking of the surface proteins in the IS (15). It seemed that only T cell cytoskeleton plays a more active role in formation of IS (16). However, recent works with B cells demonstrated the active participation of actin cytoskeleton network, defining that it contributes to cell activation and antigen presentation (17-20). Moreover, Al-Alwan et al. (21) reported that actin cytoskeleton of dendritic cells as well participates in IS formation, though the mechanism of T cell activation appeared to differ from activation with B cells (22).
T cell interaction with APC
The first point to consider in the interaction of T cells and APCs is that their surfaces are covered by glycocalyx, consisting of "huge" glycoproteins like CD45 or CD43 on T cells (23). It provides cells quite rigid protective layer that hinders relatively small surface molecules such as TCR or MHC (24-26). Moreover, there also exists a repellent power between two cells because of their negative charges on the surface (27). How do cells overcome these obstacles?
Ueda et al. have distinguished four different stages that Ag-specific T helper cell overcomes the obstacles during synapse formation with APCs (B cell line CH27 and freshly isolated dendritic cells). Using high resolution microscopy methods they visualized cells from the earliest time of interaction to 6 h. The most intriguing event that they saw was the formation of invasive pseudopodia in the first stages of synapse formation. Interestingly, this penetration did not damage the membrane neither in T cell nor in APC, even though T cell pseudopodia almost reached the nuclear envelope of target cell. Authors speculate that this event might help to increase the surface area between two cells raising the possibility to make more TCR-peptide-MHC (major histocompatibility complex on APCs) conjugates and perform full response. The second stage is a transition between 1 and 3 stages, during which microtubule network is actively formed near the contact site of T cell and APC. During third stage centrioles are positioned proximally to the contact zone, whereas in the last stage the enlargement of Golgi apparatus was seen (28) (Fig. 1).
Implementation of biomembrane force probe and a model APC enabled scientists to perform quantitative characterization of mechanical forces exerted by T cells upon engagement of TCR and/or lymphocyte function-associated antigen-1 (LFA-1). It was shown that T cells establish sequential pushing and pulling forces against their target after conjugation of TCR. The cells adapted the force amount depending on rigidity of the target (29). Even though LFA-1 molecules play important role in T cell and APC activation and IS formation (2,30,31), in these experiments, LFA-1 conjugation alone did not induce force generation in T cells (29). On the other hand, LFA-1 was indispensable for interaction forces between T cells and APCs, with the peak of force corresponding the maximal formation of IS (32). Recently, more papers report about mechanosensitivity of TCR, suggesting that it converts mechanical energy to chemical signalling (33,34). Judokusumo et al. used polyacrylamide gels with immobilized ligands to CD3 and/or CD28 to demonstrate how rigidity of the gel (varying elastic modulus from 10 to 200 kPa) affects the attachment and response of the T cells. Cells exhibited better attachment and activation on more rigid substrates and mechanosensitivity seemed to be more dependent on CD3 rather than CD28 signalling. It was shown that the sensitivity to substrate rigidity was partially mediated by TCR downstream signalling (35). Although some controversial results suggest that physiological aspect of effective IS formation still needs further research, as of now, it seems probable that TCR might be one of the main elements, which is indispensable for induction of forces generated during initial states of T cell invasive protrusion.
Signalling cascades that induces actin cytoskeleton rearrangement
Once T cell manages to get conjugated with specific peptide-MHC complexes on APC surface, downstream signal is relayed. The initial stimulation through TCR/CD3 (TCR-associated phosphorylation) peaks within first minute of contact initiation (36-40). Then, IS starts to form and mature IS appears approximately within 30 min for naïve T cells and within 1~3 min for T cell blasts after initial T cell-APC contact (41) and might persist for as long as 20 h (11), depending on the status of T cell.
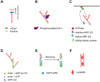
Downstream signalling starts with the activation of Src-kinases (proto-oncogene tyrosine-protein kinase - Fyn and lymphocyte-specific protein tyrosine kinase - Lck). They are recruited to the cytoplasmic site of TCR complex where they phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) on the CD3 and ζ chains. Fyn and Lck are separated spatially in lipid rafts and can be sequentially activated (42,43). The phosphorylated motifs then create docking sites for the Syk-kinase zeta chain-associated protein of 70 KDa (ZAP70). After recruitment, the latter one is then activated by Src-kinases, and in turn phosphorylates many members of TCR-proximal activation complex. Two of the ZAP70 targets are linker for the activation of T cells (16,44) and haematopoietic-cell-specific protein-1 (HS-1) (45,46). Phosphorylated LAT then recruits different proteins through its SRC homology-2 domains, among which are PLC-γ1 (phospholipase C γ1) and adapters Gads (Grb2-like adapter downstream of Shc), Grb2 (Growth factor receptor-bound protein 2), and Grap (GRB2-related adapter protein) (47,48). Gads through its SH3 domain in turn attracts SRC homology-2 (SH2)-domain-containing leukocyte protein of 76 kDa (SLP76) (49). Complex with SLP76 creates additional binding platform for actin-regulatory components, such as PLC-γ1, adaptor NCK (non-catalytic region of tyrosine kinase), and ITK (interleukin-2-inducible T cell kinase), which recruits VAV1 - Rho-family GTPase nucleotide exchange factor (GEF) activating downstream GTPases (50-53). PLC-γ1 is phosphorylated by activated ITK. This pathway leads to Ca2+ release from endoplasmic reticulum. Ca2+ increment in cytoplasm is necessary for filamentous actin (F-actin) reorganization in response to TCR-crosslinking (47). Rearrangements of actin cytoskeleton are mediated by many different regulator proteins which bind to actin (Fig. 2).
Linear actin polymerization
mDIA1 and FMNL1
The small GTPase Rho participates in many actin-related processes in the cells; one of which is cell adhesion characteristic to lymphocytes (54). mDIA1 protein (mammalian homolog of Drosophila diaphanous 1) is in the downstream of RhoA small GTPase (55). This protein belongs to large formin family, which participate in unbranched nucleation of actin filaments independently from Arp2/3 (56-58). Among formins, T cells express mDIA1 and FMNL1 (Formin-like-1), both of which can bind to profilin - actin nucleating protein. These two proteins are reported to participate in MTOC polarization (in Rac1-dependent manner) and cell-mediated killing in T cells. Separately from Arp2/3 (actin-related protein 2/3) complex, they regulate the polarization of centrosome and microtubule during T cell activation (57,59). mDIA1 is rendered inactive in the complex with Diaphanous-autoregulatory domain (DAD) until it is released by RhoA GTPase (60). Mice deficient in mDIA1 show defects in T lymphocytes trafficking to secondary lymphoid organs, reduced chemotaxis, and impaired formation of actin filaments and cell polarization after chemotactic stimulation in vitro (61,62).
Actin nucleators
HS1
During T cell activation, HS1 is one of the substrates for tyrosine phosphorylation (63,64). HS1 is expressed specifically in haematopoietic cells and is related to cortactin, an actin regulatory protein (65,66). It has Arp2/3 binding domain, coiled-coil domain for F-actin binding, proline-rich domain for Lck/VAV1/PLCγ1 binding, and two phosphorylated tyrosine residues as a binding sites for ITK (46,64,67). Phosphorylation is required for HS1 recruitment to the IS. c-Abl tyrosine kinase binds to phospho-HS1 via its SH2 domains and is required for full tyrosine phosphorylation of HS1 during T cell activation (68). HS1 co-localizes with Arp2/3 complex, thereby increasing the rate of actin assembly and promoting branched actin network formation induced by Arp2/3 (69). In IS, HS1 is required for maintenance of actin responses and Ca2+ signalling. HS1-/- T cells demonstrate impaired IL-2 production resulted in the defects in NFAT and NFκB activation. Furthermore, HS1 interacts and stabilizes the action of VAV1 in T cells (45). The recruitment of HS1 to IS is mediated by ITK (46).
HS1 plays a role in APCs as well. It is phosphorylated in B cells upon the stimulation of BCR (B cell receptor) (70) and the phosphorylation is synergistically mediated by Lyn and Syk (71). Recently, some reports demonstrated HS1 role in B cell chronic lymphocytic leukaemia. HS1 plays a central role in actin cytoskeleton rearrangement in migrating cells, controlling their trafficking, homing, and promoting the tissue invasion (72,73). HS1 depleted dendritic cells show unusual lamellipodial dynamics or podosome formation and have reduced direction persistence during migration (74). Furthermore, Huang et al. observed that HS1 is as well required for correct antigen uptake and presentation by dendritic cells (75).
WASp
WASp was the first identified actin regulating protein in mammals as it was linked with Wiskott-Aldrich syndrome - an X-linked primary immunodeficiency (76). Humans with mutations in WAS gene, which encodes WASp, have low numbers of small platelets, easily get bruises, have prolonged bleeding, and may display eczema and frequent infections. There is classical form of disease caused by complete loss of WASp expression, which results in severe clinical course, whereas the mild form of a disease caused by low levels of WASp usually cause X-linked thrombocytopenia with lighter form of disease (77,78). As this protein has a strong clinical background it received lots of attention from scientists and therefore the roles were extensively analysed and reviewed (79).
WASp is expressed exceptionally in haematopoietic cell lines, but other proteins from WASp family are distributed almost in all mammalian tissues: neural WASp (N-WASp), WASp family verprolin homologous protein 1-3 (WASp1, WASp2, and WASp3), Wiskott-Aldrich syndrome protein and SCAR homologue (WASH), and WASp homologue-associated protein with actin, membranes and microtubules (WHAMM) (80,81). WASp family proteins do not bear intrinsic enzymatic activity and mainly function as scaffold proteins, relaying wide range of upstream signals to control actin cytoskeleton rearrangements. All of them have common verprolin homology/cofilin homology/acidic region (VCA) domain and some bear proline-rich domain (PRD). WASp family proteins through VCA domain in C-terminus bind complexes formed from Arp2 and Arp3. Activated Arp2/3 complexes then recruit monomeric actins, and promote nucleation of branched actin filaments (82-84).
WASp in the cytoplasm is kept in auto-inhibited form (85). Many proteins are reported to bind to WASp and modulate its affinity to Arp2/3 (79). In immune cells WASp is constitutively bound to WIP (WASp interacting protein), which hinders the Arp2/3 binding site by wrapping its C-terminal domain around the WASp-WH1 domain (WASp homology domain 1). Binding to WIP not only inhibits WASp but also is necessary for its stability. Furthermore, WIP is also a chaperone of WASp, regulating its activation and is responsible for the localization of WASp at actin rearrangement site followed by T cell activation (86-88). WIP was reported to be essential for IL-2 signalling and responsiveness in T cells (89).
Interaction of Rho-family small GTPase Cdc42 (GTP-bound) with the GTPase-binding domain in WASp releases C-terminus from the auto-inhibition. This discloses the binding site for Arp2/3 complex and that mediates actin nucleation (90). WASp can also be activated alternatively through NCK1/NCK2 within binding to SLP76 acidic domain or binding to adhesion-and degranulation-promoting adaptor protein (ADAP), which then binds to SH2 domain in SLP76 (91,92). Furthermore, dimerization/oligomerization of WASp can increase the affinity to Arp2/3 (93). In T cells, there are also alternative ways to regulate WASp after TCR ligation with peptide-MHC complex. Phosphorylation of tyrosine residue Y291 by FYN and dephosphorylation by protein tyrosine phosphatase (PTP)-PEST are known to regulate the activity of WASp. Both of these events may occur independently of Cdc42 binding and are essential for WASp activity during T cell activation (94).
WASp knockout mice share similar symptoms with human disease, such as defects in lymphocyte migration and podosome formation, defects in T cell and B cell signalling, failure to generate effective immune responses and development of autoimmunity (95-98). T cells deficient in WASp exhibit abnormal smooth cell membranes and are defective in proliferation and actin polymerization (99). WASp deficiency in B cells has been reported to be the major cause of systematic autoimmunity developed in Wiskott-Aldrich syndrome. WASp-/- B cells are hyperresponsive to BCR and Toll-like receptor signals in vitro, which then promotes the loss of tolerance (100). Meanwhile, dendritic cells seem to employ WASp directly for synapse formation and T-cell priming. WASp-deficient dendritic cells form less stable contacts with T cells, thereby causing multiple defects of IS structure and further defective function of T cells (101). This suggest that actin cytoskeleton rearrangements play active role in successful IS formation in both cells.
WAVE2
There are three isoforms of WAVE, two of which (WAVE1 and 3) are mainly expressed in central nervous system cells, whereas WAVE2 is in hematopoietic cells (102). Rho family GTPases are critical signal transducers, which transmit the signals from cell membrane to the cytoskeleton and cell adhesions (103). In T cells, downstream signal of TCR stimulation that leads to the cytoskeleton rearrangements is mainly relayed through three GTPases - RhoA, Cdc42, and Rac1. In an earlier part of this review, we discussed the effector molecules of RhoA and Cdc42 effector molecules: mDia1 and WASp, respectively. Rac1 is an upstream regulator of WAVE together with NCK (104,105).
WAVE proteins have WAVE homology domain (WHD) at their N-terminus, followed by a basic region (BR), proline-rich region (PRR), and VCA region at C-terminus. As mentioned before, VCA domain is necessary for binding actin and Arp2/3 complex. Contrary to WASp, WAVE2 is not auto-inhibited, but exists in a multimolecular complex with other proteins including Abi1 and 2, HEM1 (NAP1), PIR121 (SRA1), and HSPC300 (105,106). Activated Rac1 interacts with PIR121 and therefore recruits WAVE2 to the areas of cellular activation, where WAVE2 then participates in actin polymerization (106). The recruitment of WAVE2 to IS seems to be dependent on tyrosine phosphorylation at Y150 by c-Abl tyrosine kinase (mediated by Abi1/2), which also increases actin polymerization activity (68,107).
In T cells WAVE2 is shown to be important during IS formation. After TCR activation, WAVE2 is responsible for regulating the actin reorganization at the contact zone of T cell and APC. WAVE2-suppressed T cells practically show little or no F-actin at the IS (WASp-suppressed T cells are comparable with WT, not exhibiting any defects in F-actin polymerization near the contact zone). Furthermore, WAVE2 mediates integrin function, and CRAC-mediated Ca2+ entry (independent form PLCγ1 pathway), which leads to full T cell activation (108). In natural killer (NK) cells, WASp is necessary for F-actin accumulation at the IS and cytotoxicity. A recent report showed that function of WASp-deficient NK cells can be restored by IL-2 stimulation as this cytokine induces WAVE2 WAVE2-mediated actin polymerization at the IS (109). This demonstrates the importance of overlapping regulation of actin cytoskeleton in the cells. Interestingly, WAVE2 deficient mice die during gestation displaying defects in development and Rac1-mediated cell migration (110,111).
WASH
WASH protein was identified quite recently. It is
a homologue of WASp and SCAR and is evolutionary conserved. WASp is widely expressed in mammalian tissues and cell lines and colocalizes with actin in filopodia and lamellipodia. As other members of WASp family, WASH contains VCA domain, through which it binds to Arp2/3 complex and promotes actin polymerization. Although WASp or SCAR proteins bear two evolutionary conserved regions, WASH homology domains 1 and 2 (WHD1 and 2), WASH lacks these regions in N-terminus (80). WHD1 facilitates WASH distribution in the cell and interaction with other proteins. WASH also has a tubulin-binding region (TBR), which enables the Rho GTPase-regulated association with microtubules (58). WASH has been shown to localize with early recycling endosomes and mediate their retromer-dependent sorting through Arp2/3 activity (112,113). Surprisingly, some data show that Arp2/3 complex binding to WASH suppress its ability to crosslink (bundle) F-actin (mediated by Rho1) and, therefore, promote branched actin nucleation (114). It is not known yet whether WASH has a function in IS formation. WASH and WAVE complexes appear to be structurally similar, so they are controlled by structurally related mechanisms (115). This suggests that these proteins might have an overlapping function which is yet to be discovered.
Branched actin polymerization
Arp2/3
Arp2/3 is the first major identified actin nucleator. It is highly conserved practically in all eukaryotic organisms. It is a complex of seven stably associated polypeptides, including Arp2 and Arp3. Namely, Arp2/3 complex is known to have actin nucleating activity and ability to organize actin filaments into branched networks (58). Arp2 and Arp3 are structurally similar to actin (116). Arp2/3 complex alone cannot nucleate actin filaments, although binding to filament increases its activity. Potent nucleation requires phosphorylation of Thr and Tyr residues in Arp2. Third element that is necessary for complex activation is the nucleation-promoting factors (NPFs). Engagement with NFP through their VCA domain (discussed earlier) leads to conformational change (e.g., WASp, WAVE or WASH) needed to initiate the nucleation (58). NPFs are classified into two groups, class I and class II based on their activation mechanisms. NPFs activated after triggering of TCR belong to the class I NPF The main most basic model of a strategy used by class I NPFs is that their VCA domain delivers an actin monomer to the complex, forming a trimer of Arp2, Arp3 and actin, which further functions as the nucleus for a new actin filament. The acidic domain of NFP is reported to mediate binding to the Arp2/3 complex, while central region of NFP initiates the activating conformational change in the complex. When y-branch is formed, nucleation-promoting factors dissociate from the Arp2/3 complex and can again participate in branch formation (117). Capping protein together with profilin act synergistically with Arp2/3 complex to favour the nucleation of branched actin network. Aged actin filaments release phosphate at the minus end, causing the dissociation of Arp2/3 complex from the pointed ends of filaments. These kinds of properties provide actin network mobile and rigid characteristics at the same time (82).
T cells lacking Arp2/3 complex form filopodial protrusions rather than lamellipodia, after meeting APC. This most probably is the cause of decrease in the number of T cell-APC conjugate formation, defective integrin activation and TCR internalization (59). On the other hand, formation of filopodial structures in the contact site with APC signifies that T cell use not only the branched actin network for efficient IS formation (118).
Actin depolymerization
For actin engine to work efficiently, turnover of actin filament should be very effective. Actin depolymerization occurs passively in the ageing end of actin filament and is regulated by actin-depolymerizing factor (ADF) and cofilins. All eukaryotes express these small actin-binding proteins. Mammalian ADF and cofilin-1 (nonmuscle) boost the dynamics of cytoskeletal network by depolymerizing actin filaments in minus ends and promoting actin turnover (119). In T cells, not TCR signaling but co-stimulation by CD2, CD4, CD8 or CD28 induces the phosphorylation and, therefore, activation of cofilin, which then associates with the cytoskeleton (120).
Other cytoskeleton related factors that play roles in the formation or sustention of IS
Dynamin-2 (Dyn2) is a large GTPase (only one isoform of dynamin found in hematopoietic cell line) which affects TCR-stimulated T cell activation by regulating multiple distal signaling pathways (Erk, Jnk, and PLCγ1) and the accumulation of F-actin at the immunological synapse. Dyn2 was also reported to interact with VAV1 during the activation, but does not participate in the regulation of LAT complex formation. Precisely, through interaction with VAV1, Dyn2 can exert its regulating functions, as T cells lacking Dyn2 exhibit similar characteristics to those lacking VAV1 (121).
Adhesive interactions by integrins during IS formation and sustention are crucial for full T cell activation. CD2-associated protein (CD2AP) is an adaptor protein, which stabilizes contacts between T cell and APC, by linking CD2 adhesion molecule with actin cytoskeleton. Binding of CD2AP to cytoplasmic domain of CD2 molecule is mediated by TCR stimulation (122). The most important adhesion molecule in T cell in the interface with APC is considered to be a LFA-1 (leukocyte function-associated antigen-1; its ligand is ICAM-1 on APC). In inactivated T cells, LFA-1 is held in the state of low affinity to its ligand. However, during TCR-peptide-MHC ligation, integrins undergo conformational changes mediated by cytoplasmic proteins which link integrins with cytoskeleton. This process increases their affinity and avidity to their ligands. The detailed pathways of LFA-1 activation are reviewed recently (123).
Endosomal clathrin, which mainly was thought to participate in endocytic processes, has been reported to accumulate and regulate actin rearrangements at the IS in T cells. Clathrin depleted T cells are unable to accumulate actin or proteins involved in actin polymerization, such as dynamin-2, Arp2/3 complex and CD2AP to IS. Thus, clathrin might serve as a platform bringing some necessary elements for actin reassembling at the contact site between T cell and APC (124).
Previously, we mentioned that T cells lacking Arp2/3 complex do form filopodia at the contact site with APC. Thus, we suggest that both branched network and nonbranched long arrays contribute for the formation of immunological synapse. Wang et al. reported that actin bundling protein L-plastin is a positive regulator of TCR-mediated cytokine production and cell proliferation. L-plastin has two sites for actin binding; therefore, it can aggregate actin filaments into parallel bundles (125). L-plastin deficient T cells form smaller synapses, which are less stable, causing defects in effective T cell stimulation (125,126).
IS formation and actin cytoskeleton
IS formation is not a simple task for the T cells to achieve. If it is the synapse between naïve T cell and APC, it has to persist for a relatively long time. Although repeated IS with intermittent signal was shown to be sufficient for T cell activation (127), stable contacts may play a stronger role in adaptive immune system.
The first step that T cell should make after meeting APC is stopping their migration. One of the recent models is based on increased Rac1 activity and inhibition of RhoA, which leads to decreased ERM phosphorylation, increased stathmin phosphorylation and loss of stable uropod together with formation of multiple lamellipodial protrusions (128). Upon the engagement of TCR, not only does T cell stop, but it also starts to reorganize its actin cytoskeleton. The radial actin polymerization forms a lamellipodia over the APC that way increasing the contact area. After that area reaches its maximal size, the F-actin layer beneath the contact zone continues to undergo polymerization at the edge, which results in centripetal actin flow. From this moment, the second layer of F-actin on top of the first one is formed. Force-generated signalling events during their interaction lead to stabilization of integrin-mediated adhesion. The lower actin layer forms lamella, whereas the upper one forms lamellipodium (9).
A few seconds after recognition of peptide-MHC, TCRs start forming larger oligomers - microclusters. These microclusters are associated with co-stimulatory receptors (CD28), Lck and ZAP70, and serine kinases - protein kinase C (PKCθ) and adaptor molecules (LAT, SLP76). During the centripetal movement of the actin, TCR microclusters move from periphery to the centre of the contact releasing kinases and adaptors and forming cSMAC (129).
Actin is exceptionally depleted from cSMAC. Therefore, actin flow-mediated TCR microcluster transport form periphery to the centre is gradually overtaken by dynein and microtubules. Microtubules are positioned near the cell membrane so that TCR microclusters move via dynein-dependent manner towards the cSMAC (130). In cSMAC receptors are targeted for degradation (131). Finally, IS is terminated when its symmetry is destroyed (132).
CONCLUSION
Understanding the mechanisms of the IS formation between T cells and APCs become clearer during the past 10 years using advanced experimental approaches. Biochemical data, high resolution imaging and biophysical view enable us to collect the information about IS at multiple levels. However, the exact connection between TCR signalling as mechanotransducer and actin cytoskeleton reorganization is still not fully understood. Further research and sophisticated interpretation of data from different points of view will let us disclose the nature of IS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download