Abstract
Background
Therapeutic approaches using monoclonal antibodies (mAbs) against complement regulatory proteins (CRPs:i.e.,CD46,CD55 and CD59) have been reported for adjuvant cancer therapy. In this study, we generated a recombinant 1E8 single-chain anti-CD59 antibody (scFv-Fc) and tested anti-cancer effect.by using complement dependent cytotoxicity (CDC).
Methods
We isolated mRNA from 1E8 hybridoma cells and amplified the variable regions of the heavy chain (VH) and light chain (VL) genes using reverse-transcriptase polymerase chain reaction (RT-PCR). Using a linker, the amplified sequences for the heavy and light chains were each connected to the sequence for a single polypeptide chain that was designed to be expressed. The VL and VH fragments were cloned into the pOptiVEC-TOPO vector that contained the human CH2-CH3 fragment. Then, 293T cells were transfected with the 1E8 single-chain Fv-Fc (scFv-Fc) constructs. CD59 expression was evaluated in the prostate cancer cell lines using flow cytometry. The enhancement of CDC effect by mouse 1E8 and 1E8 scFv-Fc were evaluated using a cytotoxicity assay.
Results
The scFv-Fc constructs were expressed by the transfected 293T cells and secreted into the culture medium. The immunoreactivity of the secreted scFv-Fc construct was similar to that of the mouse 1E8 for CCRF-CEM cells. The molecular masses of 1E8 scFv-Fc were about 120 kDa and 55 kDa under reducing and non-reducing conditions, respectively. The DNA sequence of 1E8 scFv-Fc was obtained and presented. CD59 was highly expressed by the prostate cancer cell line. The recombinant 1E8 scFv-Fc mAb revealed significantly enhanced CDC effect similar with mouse 1E8 for prostate cancer cells.
The complement system is an essential component of innate immunity and is involved in host defense against micro-organisms and other infectious agents (1,2). However, the activated components of complement produced during inflammation and immune responses can attack the host cells in the absence of a system of protection (3). Membrane-bound complement-regulatory proteins (CRPs), including CD55, CD59, and CD46, play roles in this process. As the CRPs are expressed in almost all human tissues and are able to inactivate complement components, under normal conditions there is no bystander effect from circulating complement (4,5).
Among the CRPs, human protectin (CD59) is an 18~25-kDa glycoprotein that is linked to the cell membrane via a glycosylphosphatidylinositol (GPI) anchor. CD59 inhibits complement-mediated lysis by preventing the full assembly of the membrane attack complex (MAC) on host cells. It binds to C8 in the C5b-8 complex, preventing the polymerization of C9 during the final step of MAC formation (6-10). Inhibition of CD59 function by an anti-CD59 mAb sensitizes tumor cells to complement attack (11-15). In addition, CD59-neutralizing antibodies enhance the antitumor activities of rituximab and herceptin against lymphoma cells and lung cancer cells (16,17).
In the mid-1980s, single-chain Fv (scFv) constructs, in which the isolated antibody variable heavy chain (VH) and variable light chain (VL) domains are joined by a flexible linker of 15~20 amino acids, were developed. The small size of scFv, its rapid clearance from the circulation, and its tumor-penetrating properties make it a promising candidate for targeting tumors (16-22). Recently, the scFv-Fc strategy has become one of the most popular methods in antibody engineering, offering numerous advantages over traditional methods (23).
In the present study, using a previously established hybridoma clone that produces a novel mAb (1E8) against human CD59, we cloned the 1E8 scVH and scVL genes, respectively. In addition, we generated a 1E8 scFv-Fc recombinant mAb and evaluated its CDC effects in prostatic cancer cell line.
The prostate cancer cell line PC3 was maintained in Dulbecco's modified Eagle's medium (DMEM) that was supplemented with 10% fetal bovine serum (FBS). Rabbit complement (Cedarlane, Burlington, ON, Canada) was reconstituted in 1 ml of ice-cold distilled water, passed through a filter with pore size of 0.22µm, and stored at -20℃ until use. The EZ-Cytox Cell Viability Assay Kit (Daeil Lab, Seoul, Korea) was used.
The PC3 cells were seeded onto culture plates and allowed to adhere for 24 h. Cells (106) were incubated with saturating amounts of the purified mouse 1E8 or a control immunoglobulin for 30 min at 4℃. Thereafter, the cells were washed twice with phosphate-buffered saline (PBS), incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody for 20 min at 4℃, and washed twice with PBS. The cells were fixed with 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA). Cells were analyzed in a FACS apparatus (Beckman Coulter, Brea, CA, USA).
PC3 cells (5×103) in 100µl of DMEM supplemented with 10% FBS were seeded into a 96-well plate (SPL Lifesciences, Pocheon, Korea). The plates were incubated overnight at 37℃ in an atmosphere of 5% CO2, to allow the cells to adhere to the plate. The culture supernatant was removed, and the antibody (dissolved in serum-free DMEM at a concentration of 10µg/ml), either alone or in combination with 5% rabbit complement, was added to the cells. Cells in fresh medium without antibody or complement were used as the control. The plates were incubated for 3 h at 37℃. After the incubation period, 10µl of the solution from the EZ-Cytox Cell Viability Assay kit were added to each well and incubated at 37℃ in 5% CO2 for 4 h. The extent of the reduction to formazan within the cells was quantified by measuring the absorbance at 450 nm in an ELISA reader.
The hybridoma cell line RV1E8, which produces anti-CD59 antibody, was cultured in RPMI medium that contained 10% heat-inactivated FBS (GIBCO). The culture was incubated under at 37℃ in 5% CO2. After the cells had grown to a density of 106 cell, total RNA was purified using the Easy-BLUE RNA Extraction kit (iNtRON Biotechnology, Daejon, Korea).
The genes that encode the variable heavy and light chains of the anti-CD59 antibody were derived using multiple overlap polymerase chain reaction (PCR). The sequences of the variable segments of the heavy and light chains were amplified using: the sense primers 5'-TCCTCCTCTGGTGGCGGTGGCTCGGGCGGTGGTGGGCAGGTGCAGCTGAAG-3' (VH) and 5'-GGTTCCACTGGTGACGTGGCCCAGGCGGCCGACATCCAG ATGACT-3' (VL); and the antisense primer: 5'-AGATTTGGGCTCAGCGGCCCCACCGGCCCCTGAGGAGACGGTGAC-3' (VH) and 5'-ACCGCCACCAGAGGAGGAAGATCTAGAGGAACCACCTCTGATTTCCAACTT-3' (VL), so as to introduce SfiI restriction enzyme sites (underlined). The amplified VH and VL genes were assembled into the scFv gene using a linker. We used the VL-F and VH-R primers that contained SfiI sites in the PCR reaction (for a total of 40 cycles of 1 min at 94℃, 1 min at 60℃, and 1.5 min at 72℃), and purified the amplicons using the QIAquick gel extraction kit (Qiagen, Hilden, Germany), and confirmed by DNA sequencing (Cosmo Genetech, Seoul, Korea). The scFv DNA products were digested with SfiI, gel-purified, and then ligated into the pOptiVEC-TOPO expression vector (Dinona, Seoul, Korea), which contains the human CH2-CH3 fragment.
The day before transfection, 293T cells was cultured at 37℃ in 5% CO2 to a density of about 5×105 cells/ml in 6-well plates in DMEM medium that contained 10% heat-inactivated FBS. The cultures were 80% confluent on the day of transfection. The scFv vector was transfected into 293T cells using the Effectene transfection reagent (Qiagen). After incubation for 3 days, the culture supernatant was tested by flow cytometry.
The supernatant of the transiently transfected cells was applied to a recombinant protein column (GE Healthcare, Uppsala, Sweden) that had been previously equilibrated with binding buffer (20 mM sodium phosphate [pH 7.2], 0.15 M NaCl). The column was washed with binding buffer, and protein was eluted with elution buffer (0.1 M glycine-HCl [pH 3.0]). The eluted fractions were collected in tubes that contained neutralizing buffer (1 M Tris-HCl, [pH 8.0]), so as to adjust the pH to approximately 7. Finally, the purified samples were dialyzed against PBS. The purity of the eluted antibody fraction was analyzed by SDS-PAGE on 10% gels under reducing or non-reducing conditions. Protein bands were visualized by Coomassie blue staining. After electrophoresis, the proteins in the gel were transferred to a nitrocellulose membrane (Whatman, Dassel, Germany), which was then blocked with 5% skim milk in PBS. The membrane was incubated with a 1:5,000 dilution of HRP-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, Baltimore, MD, USA) for 1 h at room temperature, and then washed four times with PBST (PBS with 0.05% tween 20). Thereafter, the immunoreactive bands were visualized using an enhanced chemiluminescence detection system (ECL; Amersham Pharmacia Biotech, Uppsala, Sweden).
The VH and VL DNA fragments of mouse 1E8 were successfully amplified with the VH-F/R and VL-F/R primers, respectively. The size of the amplified VH DNA was 351 bp and that of the amplified VL DNA was 327 bp. Using VL and VH DNA, a 781bp DNA were overlapped by linking peptide inserted in the middle of VL and VH (Fig. 1A). The purified 1E8-scFv gene fragment was digested with SfiI and cloned into the pOptiVEC-TOPO transfer vector. The ligation product was transformed into competent cells and positive colonies were selected after incubation at 37℃. Plasmid DNA was extracted using a Mini-Prep kit (Qiagen). A positive clone was identified by restriction enzyme digestion (Fig. 1B). The sequencing results showed that 1E8-scFv contained the linking peptide (Fig. 2).
The constructs derived from subcloning into the pOptiVEC-TOPO vector were transfected into 293T cells using the Effectene transfection reagent. The supernatant of the transfected culture was tested for reactivity with CCRF-CEM cells by flow cytometry. The positive binding rates of the mouse 1E8 and 1E8 scFv-Fc were 97.8% and 98.8%, respectively (Fig. 3).
The recombinant antibody contained the human Fc fragment. Antibody from the supernatant was successfully purified in one step by affinity chromatography using an immobilized protein A column. The purified 1E8 scFv-Fc was analyzed by SDS-PAGE and Western blotting. SDS-PAGE disclosed discreate bands of 120 kDa at elution fraction (Fig. 4A). SDS-PAGE under reducing conditions revealed a single band of ~55 kDa. The molecular mass of the dimeric scFv-Fc under non-reducing conditions was ~120 kDa (Fig. 4B).
To examine the expression of CD59 antigens by prostate cancer cells, we assessed the binding of mouse 1E8 and 1E8 scFv-Fc on PC3 cells by FACS analysis. The mouse 1E8 and 1E8 scFv-Fc well recognized cell surface CD59 on PC3 cells (Fig. 5A). To compare the neutralizing activity of mouse 1E8 and 1E8 scFv-Fc, we tested the susceptibility of PC3 to complement-mediated damage. Approximately 20% and 25%, respectively, of the PC3 cells were sensitized by mouse 1E8 and 1E8 scFv-Fc to killing by complement (Fig. 5B).
In the present study, using a previously established hybridoma clone that produces a novel mAb (1E8) directed against human CD59, we cloned the 1E8 scVH and scVL genes. We generated a 1E8 scFv-Fc recombinant antibody and obtained the nucleotide sequence of the construct. In addition, we revealed that this recombinant 1E8 scFv-Fc mAb enhance CDC effect in CD59 expressing prostate cancer cell line.
The well known advantage of single chain minibody containing only CH2 and CH3 domain is that the scFv-Fc minibody easily penetrates solid tumors as it is smaller than the intact full chimeric antibody. Recently, it has been reported that anti-CD20 scFv-Fc (TRU-015) is more effective than rituximab in vivo against lymphoma. A clinical trial of TRU-015 for the treatment of lymphoma and inflammatory disease is in progress (23). However, scFv-Fc minibody themselves have limited usage as tumor-targeting agents in vivo, generally demonstrateing very rapid clearance from the circulation in animal models and revealing unstable characteristics during large scale production. (24,25). For the scFv-Fc minibody to be used as a therapeutic antibody, the antibody production problem would have to be resolved and its effectiveness would have to be proven in animal experiments.
We describe a method for engineering mouse 1E8 through genetic fusion of 1E8 scFv to the human CH2-CH3 domains. We obtained and presented the nucleotide sequence of 1E8-scFv (Fig. 2). Currently, this DNA sequence of mAb against CD59 can not be easily available in the public domain. We found that the level of immunoreactivity of scFv-Fc was similar to that of the mouse 1E8 mAb (Fig. 3). This implies that the constructed 1E8 scFv-Fc is structurally stable and bind effectively. The recombinant 1E8 scFv-Fc was isolated from the culture supernatants by protein A affinity chromatography and analyzed. The results of the SDS-PAGE and Western blot analyses showed discrete bands of expected molecular size, so the product was a monomer under reducing conditions but formed a homodimer under non-reducing conditions (Fig. 4). The large molecular mass of the product in the non-reducing condition is presumed to reflect glycosylation. These results suggest that 1E8 scFv-Fc is a dimeric antibody, similar to the intact antibody. This could be an important factor for the structural stability of 1E8 scFv-Fc.
The complement cascade is an effector system that is called into action by a therapeutic mAb. However, the over-expression of complement regulatory proteins by tumor cells blocks the destructive effects of complement. Recently, it was shown that the combined use of anti-CD59 antibodies and rituximab diminished the resistance of B lymphoma cells to complement-mediated killing, and the combination of anti-CD59 antibodies and herceptin was found to be effective for enhancing the complement-mediated killing of lung cancer cells (16,17). Interestingly, it has been reported that CD59 is over-expressed by prostate cancer cells and is linked to the progress and the prognosis of prostate cancer (14). In the present study, we demonstrated the enhancement of CDC by an anti-CD59 monoclonal antibody (mouse 1E8). Over-expression of CD59 was demonstrated (Fig. 5A) in the prostate cancer cell line PC3, and CDC was shown to be induced by the combination of rabbit complement with mouse 1E8 and 1E8 scFv-Fc (Fig. 5B). This implies that recombinant 1E8 scFv-Fc antibody effectively control the CDC-inhibitory functions of CD59 by binding functionally active domain of CD59.
In the present study, the usefulness of 1E8 scFv-Fc minibody in combating prostate cancer was evaluated as a possible adjvant cancer therapeutic agent. The recombinant 1E8 antibody therapy in combination with anti-cancer drugs is under investigation in our laboratoy.
Figures and Tables
 | Figure 1Generation of the 1E8 scFv. (A) Amplification of the VH and VL genes from hybridoma 1E8 cells. Lane M, 100-bp DNA ladder; lane 1, VH PCR product; lane 2, VL PCR product; lane 3, VL-linker-VH PCR product. (B) Detection of the recombinant expression vector pOptiVEC-TOPO with 1E8 scFv-Fc. Lane M1, 1-kb DNA ladder; lane 4, construct digested with SfiI. |
 | Figure 2Nucleotide sequence and its deduced amino acid sequence of the 1E8-scFv. The shadowed part represents the linker. |
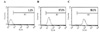 | Figure 3Immunoreactivity of the anti-CD59 scFv-Fc for CCRF-CEM cells. The flow cytometry histogram demonstrates that 1E8 scFv-Fc binds to CCRF-CEM cells. (A) Negative control (FITC-labeled human IgG); (B) positive control (FITC-labeled mouse 1E8); (C) 1E8-scFv (FITC-labeled goat anti-human IgG). |
 | Figure 4Identification of purified recombinant 1E8 scFv-Fc on SDS-PAGE. Anti-CD59 scFv-Fc was purified using an immobilized protein A column and detected by Western blotting (A) and Coomassie blue staining (B). M, marker; LS, supernatant of the transfectant; FT, after Mabselect Sure purification; #1-3, washing buffer; #4-7, elution fraction; R, reducing conditions; NR, non-reducing conditions. Note clear bands of 120 kDa at elusion fraction (#5-7) and of 120 kDa at non-reducing and 55 kDa at reducing condition. |
 | Figure 5Effects of 1E8 scFv-Fc on complement-dependent cytotoxicity for CD59-expressing PC3 cells. (A) CD59 is highly expressed on PC3 cells. (B) 1E8 scFv-Fc enhances complement-dependent cytotoxicity in the presence of rabbit complement (RC) showing similar effect with mouse 1E8. negative control (IgG); mouse 1E8 (1E8). The numbers of viable cells were evaluated in a cytotoxicity assay. |
References
1. Ramaglia V, King RH, Nourallah M, Wolterman R, de Jonge R, Ramkema M, Vigar MA, van der Wetering S, Morgan BP, Troost D, Baas F. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J Neurosci. 2007. 27:7663–7672.

2. Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, Bronson RR, Shi GP, Rother RP, Halperin JA, Qin X. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res. 2009. 104:550–558.

3. Ziller F, Macor P, Bulla R, Sblattero D, Marzari R, Tedesco F. Controlling complement resistance in cancer by using human monoclonal antibodies that neutralize complement-regulatory proteins CD55 and CD59. Eur J Immunol. 2005. 35:2175–2183.

4. Buettner R, Huang M, Gritsko T, Karras J, Enkemann S, Mesa T, Nam S, Yu H, Jove R. Activated signal transducers and activators of transcription 3 signaling induces CD46 expression and protects human cancer cells from complement-dependent cytotoxicity. Mol Cancer Res. 2007. 5:823–832.

5. Shi XX, Zhang B, Zang JL, Wang GY, Gao MH. CD59 silencing via retrovirus-mediated RNA interference enhanced complement-mediated cell damage in ovary cancer. Cell Mol Immunol. 2009. 6:61–66.

6. Geis N, Zell S, Rutz R, Li W, Giese T, Mamidi S, Schultz S, Kirschfink M. Inhibition of membrane complement inhibitor expression (CD46, CD55, CD59) by siRNA sensitizes tumor cells to complement attack in vitro. Curr Cancer Drug Targets. 2010. 10:922–931.

7. Yan J, Allendorf DJ, Li B, Yan R, Hansen R, Donev R. The role of membrane complement regulatory proteins in cancer immunotherapy. Adv Exp Med Biol. 2008. 632:159–174.

8. Rushmere NK, Knowlden JM, Gee JM, Harper ME, Robertson JF, Morgan BP, Nicholson RI. Analysis of the level of mRNA expression of the membrane regulators of complement, CD59, CD55 and CD46, in breast cancer. Int J Cancer. 2004. 108:930–936.

9. Jarvis GA, Li J, Hakulinen J, Brady KA, Nordling S, Dahiya R, Meri S. Expression and function of the complement membrane attack complex inhibitor protectin (CD59) in human prostate cancer. Int J Cancer. 1997. 71:1049–1055.

10. Sivasankar B, Longhi MP, Gallagher KM, Betts GJ, Morgan BP, Godkin AJ, Gallimore AM. CD59 blockade enhances antigen-specific CD4+ T cell responses in humans: a new target for cancer immunotherapy? J Immunol. 2009. 182:5203–5207.

11. Hu W, Yu Q, Hu N, Byrd D, Amet T, Shikuma C, Shiramizu B, Halperin JA, Qin X. A high-affinity inhibitor of human CD59 enhances complement-mediated virolysis of HIV-1: implications for treatment of HIV-1/AIDS. J Immunol. 2010. 184:359–368.

12. Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991. 65:532–537.

13. Babiker AA, Nilsson B, Ronquist G, Carlsson L, Ekdahl KN. Transfer of functional prostasomal CD59 of metastatic prostatic cancer cell origin protects cells against complement attack. Prostate. 2005. 62:105–114.

14. Xu C, Jung M, Burkhardt M, Stephan C, Schnorr D, Loening S, Jung K, Dietel M, Kristiansen G. Increased CD59 protein expression predicts a PSA relapse in patients after radical prostatectomy. Prostate. 2005. 62:224–232.

15. Watson NF, Durrant LG, Madjd Z, Ellis IO, Scholefield JH, Spendlove I. Expression of the membrane complement regulatory protein CD59 (protectin) is associated with reduced survival in colorectal cancer patients. Cancer Immunol Immunother. 2006. 55:973–980.

16. Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, Amadori A, Tedesco F. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007. 67:10556–10663.

17. Zhao WP, Zhu B, Duan YZ, Chen ZT. Neutralization of complement regulatory proteins CD55 and CD59 augments therapeutic effect of herceptin against lung carcinoma cells. Oncol Rep. 2009. 21:1405–1411.

18. Imai M, Landen C, Ohta R, Cheung NK, Tomlinson S. Complement-mediated mechanisms in anti-GD2 monoclonal antibody therapy of murine metastatic cancer. Cancer Res. 2005. 65:10562–10568.

19. Vaughan TJ, Osbourn JK, Tempest PR. Human antibodies by design. Nat Biotechnol. 1998. 16:535–539.

20. Hoogenboom HR, Chames P. Natural and designer binding sites made by phage display technology. Immunol Today. 2000. 21:371–378.

21. Choi GH, Lee DH, Min WK, Cho YJ, Kweon DH, Son DH, Park K, Seo JH. Cloning, expression, and characterization of single-chain variable fragment antibody against mycotoxin deoxynivalenol in recombinant Escherichia coli. Protein Expr Purif. 2004. 35:84–92.

22. Cao M, Cao P, Yan H, Lu W, Ren F, Hu Y, Zhang S. Construction, purification, and characterization of anti-BAFF scFv-Fc fusion antibody expressed in CHO/dhfr-cells. Appl Biochem Biotechnol. 2009. 157:562–574.
23. Hayden-Ledbetter MS, Cerveny CG, Espling E, Brady WA, Grosmaire LS, Tan P, Bader R, Slater S, Nilsson CA, Barone DS, Simon A, Bradley C, Thompson PA, Wahl AF, Ledbetter JA. CD20-directed small modular immunopharmaceutical, TRU-015, depletes normal and malignant B cells. Clin Cancer Res. 2009. 15:2739–2746.
24. Choi TH, Choi CW, Awh OD, Lim SM. Expression of the recombinant single-chain anti-B cell lymphoma antibody. Korean J Biomed Lab Sci. 2003. 9:111–121.
25. Hu S, Shively L, Raubitschek A, Sherman M, Williams LE, Wong JY, Shively JE, Wu AM. Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 1996. 56:3055–3061.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download