Abstract
We previously reported that Hydnocarpi Semen (HS) has a wound healing effect on diabetic foot ulcer lesion in mice. In this study, ethylacetate (EtOAc) fraction from HS extract were evaluated for their wound healing activity by using in vitro acute inflammation model. GC and GC/MS analysis shows that the main constituents in EtOAc fraction are chaulmoogric acid, hydnocarpic acid, and gorlic acid. EtOAc fraction activated macrophages to increase the production of TNF-α. The fraction also increased the production of TGF-β and VEGF, which induced fibroblast activation and angiogenesis. These results suggest that the mechanism that the fraction helps to enhance healing of skin wound is possibly associated with the production of TNF-α, as well as secretion of VEGF, TGF-β and HS may have a new bioactive material for the treatment of skin wound.
Wound repair develops with the initiation of inflammatory responses, as inflammation itself is the primary response to tissue injury such as burn and ulcer. Acute inflammation may be resolved completely, with locally injured parenchymal elements being regenerated without a significant scar. When dermal wound is filled by a fibrin clot, inflammatory cells release transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF), which stimulate fibroblasts from the adjacent intact dermis to migrate to the wounded site and become involved in angiogenesis at wound area (1).
Herbal medicine was traditionally used for the treatment of several diseases in oriental countries such as Korea, China, and Japan for centuries. Recently, many articles based on herbal medicine have been reported with great interest (2,3). Hydnocarpi Semen (HS) has been used for the treatment of leprosy (Hansen's disease) which is a chronic disease caused by Mycobacterium leprae (3-5). Skin lesions are the primary external sign in leprosy and untreated leprosy can be progressive, causing skin damage. During our research for novel bioactive materials from plants, we reported that the HS extract has a wound healing effect in hyperglycemic mice (6,7). However, it is unknown which constituents of HS extract have the wound healing activity. In this study, a fraction of ethylacetate (EtOAc) from HS crude extract was evaluated for its wound healing activity by using in vitro acute inflammation model.
HS seeds were obtained from Kyoungdong Oriental drug store (Seoul, Korea). The EtOAc fraction was prepared as previously described (7). In brief, dried HS seeds were extracted three times in 80% methanol (MeOH) and subjected to sequential liquid-liquid extraction (LLE) with a solvent series of increasing polarity: n-hexane, EtOAc and butanol. The partitioning was performed 4 times in glass separation funnels by mixing 100 ml of solvent with the aqueous phase and shaking with the rotary shaker for 15 min, and after standing, organic phase was removed (Fig. 1). The EtOAc fraction was used for this study.
The mouse macrophage cell line, RAW 264.7 cells were cultured in DMEM (Hyclone) media supplemented with 10% fetal bovine serum (Hyclone) and antibiotics (Gibco) in a 24-well cell culture plate in 5% CO2 at 37℃. The cells were treated with the fraction at dose dependent manner. Cell culture supernatants were used for measurement of cytokines.
Cell culture supernatants were assayed for TNF-α, TGF-β, and VEGF using DuoSet ELISA kit (R&D system, Minneapolis, MN, USA) according to the manufacturer's instruction.
MTT (methythiazolyldiphenyl-tetrazolium bromide, Sigma-Aldrich Korea) was used to measure the cytotoxicity of the cells with EtOAc fraction as described previously (7). Macrophages were plated in 96-well microplate and treated with the fraction. After 24 h, the medium was removed and culture media containing MTT was added and incubated for 4 h. Absorbance was measured at 550 nm with a microplate reader (Molecular Devices, Menlo Park, CA).
GC and GC/MS analysis was performed as previously described (6). Mass detector operated in 70 V electron ionization (EI) mode. One hundred microliter of sample was injected into the GC/MS system with split-less-mode. Carrier gas (He) flow was set at 0.8 ml/min. The injection volume was 100µl in the split-less injection mode. A capillary column (HP-FFAP capillary, 30 m×0.32 mm I.D., 0.25µm film thickness; Agilent Technologies Inc, Santa Clara, CA, USA) was employed. The total chromatographic run time was 30 min; however, a subset between 330 and 1,000 s was used for the analysis of FAMEs. Distribution type of fatty acids methyl esters was investigated, m/z=74 ion chromatogram. Quantization of methyl esters of fatty acids was performed by integration of appropriate peak areas in Total Ion Chromatogram (TIC).
Our previous study showed HS extract and three fractions enhanced wound healing in diabetic ulcer lesion (6,7). However, it is unknown which constituents of HS show the wound healing activity. Three fractions were prepared from the HS crude extract again (Fig. 1) and their contents were compared. This study focused on the components and action mechanism of EtOAc fraction.
We first analyzed the components of EtOAc fraction from HS by GC and GC/MS analysis. Previous report showed that chaulmoogric acid, hydnocarpic acid, and gorlic acid are the main components of the HS total extract by GC and GC/MS analysis (6). GC analysis showed that the major constituent of fatty acid in EtOAc fraction consists of chaulmoogric acid (6.687, C18), hydnocarpic acid (9.589, C16) and gorlic acid (13.019, C18) (Fig. 2). GC-MS analysis also shows the similar results and the fraction includes chaulmoogric acid (17:08.8, C18), hydnocarpic acid (31:17.5, C16) and gorlic acid (37:15.8, C18) (Data not shown). As shown in Table I, EtOAc fraction also has chaulmoogric acid, hydnocarpic acid, and gorlic acid as the main constituents even with different content. Whereas Hydnocarpi acid, Chaulmoogric acid, and Gorlic acid contents were 51.48%, 9.06%, and 23.9%, respectively, in MeOH extract, their contents in EtOAc fraction were 48.5%, 17.56%, and 18.53%, respectively.
The wound healing is a complex cellular, physiologic, and biochemical event initiated after the stimulus of injury to tissue, and consists of acute inflammation, angiogenesis, and re-epithelialization (1). The mechanism underlying most of wound healing processes involve acute inflammation and inflammatory mediators. Along with a new formation of blood vessel, which is necessary and vital in wound repair, the migrating fibroblasts fill the wound and stimulate the formation of granulation tissue (8,9). TNF-α is a well known pro-inflammatory cytokine and has an important role in the activation of vascular endothelial cell, the induction of angiogenesis, and proliferation of fibroblast (10,11). Therefore, we examined the effect of the fraction on TNF-α production by macrophages. When RAW 264.7 cells were treated with EtOAc fraction, TNF-α production was induced and increased in macrophages (Fig. 3A).
TGF-β and VEGF are also involved in fibroblast migration and angiogenesis, respectively. We next asked the effect of HS on the production of TGF-β and VEGF. As we expected, the treatment with EtOAc fraction induced the activation of macrophages and increased the production of TGF-β and VEGF at dose dependent manner (Fig. 3B and C).
The cytotoxicity of the fraction used in this study was evaluated in RAW 264.7 cells using MTT assay and no effect on cell viability was observed at any concentrations used (data not shown).
Our result indicates that the production of TNF-α , TGF-β, and VEGF in the macrophages treated with EtOAc fraction, through an unknown mechanism, is associated with a wound healing effect. There is an important relationship of wound healing between fibroblast, keratinocytes, and resident dermal cell (12). TNF-α induced the activation of vascular endothelial cell, angiogenesis, and proliferation of fibroblast. We found that the EtOAc fraction increased the production of TNF-α in a dose-dependent manner. Our data also showed that EtOAc increased the proliferation of fibroblast and induced MMP9 activity (Data not shown). Furthermore, our results showed that the production of TGF-β and VEGF also increased in macrophages treated with EtOAc fraction (Fig. 3), suggesting that TGF-β and VEGF accelerate wound healing. The increase in re-epithelialization by VEGF-treated wounds has been reported by others (13,14). VEGF is one of the most potent angiogenesis stimulating growth factors (15). Re-epithelialization is influenced by a combination of TGF-β and VEGF (16,17).
Understanding the relation between each step involved in wound healing and natural materials was pivotal for a better study of the molecular mechanism underlying the cellular response. EtOAc fraction enhanced wound healing in ulcer lesion (7) and induced inflammation in vitro cell-based model in this study. Therefore, our results suggest that HS has some bioactive component which can be a new candidate material for the treatment of skin wound such as ulcer and burn. Our future study will be to analyze and probe each of the chemical constituents of HS except fatty acid showing the wound healing effect and thereafter establish the biochemical and molecular mechanism of their wound healing.
Figures and Tables
Figure 1
The scheme for the preparation of EtOAc fraction from Hydnocarpi Semen. This figure was adapted from a scheme in reference 7.
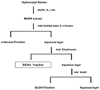
Figure 2
Typical chromatogram of fatty acids in EtOAc fraction from crude extract of Hydnocarpi Semen by GC. Each peak indicates (a) Chaulmoogric acid, (b) Hydnocarpic acid, (c) Gorlic acid.

Figure 3
The effect of EtOAc fraction on cytokine production in RAW 264.7 cell. The cells were treated with the fraction at a dose dependent manner for 24, and cell culture supernatants were collected and used for (A) TNF-α, (B) TGF-β, and (C) VEGF assay. Data are representative of at least three independent experiments, each done in triplicate; *p <0.05, **p<0.01 compared to non-treated cells.

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (2012R1A1A3012099).
References
2. Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006. 11:128–150.
3. Levy L. The activity of chaulmoogra acids against Mycobacterium leprae. Am Rev Respir Dis. 1975. 111:703–705.
4. Oommen ST, Rao M, Raju CV. Effect of oil of hydnocarpus on wound healing. Int J Lepr Other Mycobact Dis. 1999. 67:154–158.
5. Oommen ST. The effect of oil of hydnocarpus on excision wounds. Int J Lepr Other Mycobact Dis. 2000. 68:69–70.
6. Lee GS, Choi JY, Choi YJ, Yim DS, Kang TJ, Cheong JH. The wound healing effect of Hydnocarpi Semen extract on ulcer in diabetic mice. Biomol Ther. 2010. 18:329–335.

7. Lee GS, Yim D, Cheong JH, Kang TJ. The n-Hexane, ethylacetate, and butanol fractions from Hydnocarpi Semen enhanced wound healing in a mice ulcer model. Immunopharmacol Immunotoxicol. 2012. 34:968–974.

8. Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, Croitoru A, Thung S, Vlassara H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003. 52:2805–2813.

9. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007. 6:273–286.

10. Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991. 88:4220–4224.

11. Vanderslice P, Munsch CL, Rachal E, Erichsen D, Sughrue KM, Truong AN, Wygant JN, McIntyre BW, Sughrue KM, Eskin SG, Tilton RG, Polverini PJ. Angiogenesis induced by tumor necrosis factor-agr; is mediated by alpha4 integrins. Angiogenesis. 1998. 2:265–275.
12. Raghow R. The role of extracellular matrix in post inflammatory wound healing and fibrosis. FASEB J. 1994. 8:823–831.

13. Michaels J 5th, Dobryansky M, Galiano RD, Bhatt KA, Ashinoff R, Ceradini DJ, Gurtner GC. Topical vascular endothelial growth factor reverses delayed wound healing secondary to angiogenesis inhibitor administration. Wound Repair Regen. 2005. 13:506–512.

14. Saaristo A, Tammela T, Farkkilã A, Kärkkäinen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006. 169:1080–1087.

15. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001. 114:853–865.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download