Abstract
Vitamin C is an essential water-soluble nutrient which primarily exerts its effect on host defense mechanisms and immune homeostasis, but the mechanism related to immune-potentiation is poorly understood. Since dendritic cells (DCs) are known as a potent antigen presenting cell (APC) that could enhance the antigen specific immune responses, we investigate the effects of vitamin C on activation of DCs and its related mechanism by using dendritic cell lines, DC-1. First, we found that there was no damage on DC-1 by 2.5 mM of vitamin C. In the presence of vitamin C, the expression of CD80, CD86, and MHC molecules was increased, but it was decreased by the pre-treatment of SB203580, p38 MAPK-specific inhibitor. We confirmed the phosphorylation of p38 MAPK was increased by the treatment of vitamin C. Taken together, these results suggest that vitamin C could enhance the activity of dendritic cells via the up-regulation of the expression of CD80, CD86, and MHC molecules and the activation of p38 MAPK is related to this process.
Vitamin C is regarded as an essential nutrient for the host defense mechanisms through the maintenance of immune homeostasis (1,2). High concentration of vitamin C is accumulated in most immune cells including, neutrophils, macrophages, B cells and T cells, and it is rapidly decreased by infection or stress (3-6). This suggests that vitamin C acts diversely on innate immunity as well as adoptive immunity. In addition, vitamin C enhances the proliferation and migration of lymphocytes as well as the killing activity of natural killer cells (7-10). The phagocytic activity of neutrophils and macrophages is also increased by vitamin C, but the number of T cells is not altered by vitamin C (5,11). Interestingly, the decrease of delayed type hypersensitivity (DTH) to several antigens in human is caused by vitamin C insufficiency, but it is normalized by the supplementation of the sufficient amount of vitamin C (9).
It is known that vitamin C enhances Th1 immune response via the stimulation of the production of IFN-γ and TNF-α. In fact, the decreased Th1 immune response against Helicobacter pylori infection was found in vitamin C insufficient Gulo (-/-) mice, which cannot synthesize vitamin C like a human due to the lack of L-gulono-γ-lactone oxidase (12). In accordance with the up-regulation of Th1 immune responses by vitamin C, the levels of IgG1 and IgE, Th2-related isotypes of antibody, were decreased, but IgG2C, Th1-related isotype of antibody, was increased in DTH animal models (13). However, its related specific mechanism of vitamin C is still largely unknown.
Dendritic cell (DC) is known as the most potent antigen presenting cell (APC). It can readily stimulate naïve T cells, since the essential co-stimulatory molecules, such as CD80 and CD86, are highly and constitutively expressed on its surface. Immature DCs can uptake antigens and migrate to lymph nodes (13). Then, in the presence of endogenous or exogenous inflammatory signals, DCs undergo maturation (14). Maturation of DCs is related with enhanced expression of co-stimulatory molecules such as CD80 and CD86, and MHC molecules. Several kinds of pattern recognition receptor (PRR) including toll like receptor (TLR) are closely involved in the maturation and activation of dendritic cells by the stimulation of danger associated molecular pattern (DAMP) (15,16). In addition, impaired production of reactive oxygen species (ROS) during Mycobacterium tuberculosis infection affects the differentiation of DCs (17). This suggests that anti-oxidant molecules, such as vitamin C and vitamin E, could modulate the activation and differentiation of DCs.
In the present study, we investigated whether vitamin C could modulate the activation of DCs through the regulation of CD80, CD86 and MHC II expression and its related mechanisms.
Dendritic cell line, DC-1 cells were cultured in RPMI 1640 media containing 2 mM of L-glutamine, 100 units/ml of penicillin, 100 mg/ml of streptomycin, and 10% fetal bovine serum (Gibco, USA). Cells were cultured at 37℃ in a humidified atmosphere containing 5% CO2.
After cells (2×106) were exposed to various concentration of vitamin C (0.5, 1, 2 and 5 mM) for 24 hrs, they were collected and washed twice with cold PBS. Then they were resuspended in 1 × binding buffer at a concentration of 1×106 cells/ml. Cells were then incubated with 5µl of FITC conjugated Annexin V (BD Pharmingen, San Diego, CA, USA) at room temperature for 15 min in the dark. One microliter of 7-AAD (BD Pharmingen, San Diego, CA, USA) was added prior to flow cytometric analysis by FACSCaliber (BD Pharmingen, San Diego, CA, USA).
Cells (5×104) were incubated in a 96-well plate. Cells were then further incubated with 50µM of 2',7'-dichlorofluorescein diacetate (DCFH-DA; Eastman Kodak, Rochester, NY, USA) at 37℃. After incubation, cells were analyzed with a Cytofluor 2350 plate reader (Millipore, Bedford, MA, USA) with excitation at 485 nm and emission at 525 nm.
Cells (4×105) were incubated for 24 hrs in the presence of 2.5 mM of vitamin C. And then, cells were stained with FITC-conjugated antibodies against CD80, CD86, and MHC class II (BD Pharmingen, USA) on ice for 30 min. The expression levels were analyzed using flow cytometric analysis by FACSCaliber (BD Pharmingen, San Diego, CA, USA). To determine the signaling pathway by vitamin C, cells were pre-treated with specific inhibitors, 10µM of SB203580, 20µM of LY294002, and 20µ M of SP600125 for 30 min, prior to exposure to 2.5 mM of vitamin C. Then the expression of CD80, CD86 and MHC class II were analyzed using flow cytometric analysis.
The phosphorylation of p38MAPK in DC1 upon the treatment of vitamin C was examined by western blot analysis. Cells were lysed in cell lysis buffer containing 1% Triton X-100 (v/v) in 20mM Tris-HCl, pH 8.3, 150 mM NaCl and protease and phosphatase inhibitor cocktail. Cytosolic proteins were quantified using bicinchoninic (BCA) protein assay, then 30µg of samples were loaded to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) which were then transferred onto nitrocellulose membranes. The membranes were blocked in a solution of 5% skim milk in TBS-T buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.1% Tween-20). Blocked membranes were incubated with rabbit anti-mouse p38MAPK (1:1,000) or rabbit anti-mouse phosphor-p38MAPK (1:1,000) (Cell Signaling Technology, USA). Horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:2,000) (Santa Cruz Technology, USA) was used secondary anti-body. Immunodetected bands were visualized with the ECL system (Amersham Pharmacia Biotech, UK), and results were analyzed using densitometer program (Scion image).
To determine the optimal concentration of vitamin C without cytotoxicity, we examine the induction of apoptosis on DC-1 after treatment of 0, 0.5, 1, 2.5 and 5 mM of vitamin C. As shown in Fig. 1A, more than 85% of cells were viable, even with the treatment of 2.5 mM of vitamin C. However, 60.9% of cells were viable by the treatment of 5 mM of vitamin C (Fig. 1A). Next, we examined intracellular ROS levels in DC-1 after treatment of vitamin C. Without treatment of vitamin C, intracellular ROS levels were spontaneously increased in DC-1, but it was suppressed by the treatment of vitamin C. However, there were no differences on intracellular ROS levels, among the groups that exposed vitamin C (Fig. 1B). Therefore, the relatively high concentration of vitamin C did not induce apoptosis on DC-1. And it effectively acts as an anti-oxidant molecule.
It is generally known that oxidative stress was suppresses the immune responses (18). However, we have already shown that vitamin C effectively suppresses the increase of ROS levels in DC-1. Therefore, we next compared the expression levels of CD80, CD86m and MHC class II molecules on DC-1 at 24 hrs after treatment of 1 and 2.5 mM of vitamin C. Even though we could not induce those three of molecules on DC-1 cells by the treatment of 1 mM of vitamin C, we found the extensive induction of CD80, CD86 and MHC class II on DC-1 cells by the treatment of 2.5 mM of vitamin C (Fig. 2). When we considered that DC-1 shows characteristics of immature DCs, it implies that vitamin C plays an important role in the maturation of immature dendritic cells through the up-regulation of CD80, CD86 and MHC class II molecules.
Finally, we investigated which kind of signal transducing molecule is involved in the expression of CD80, CD86 and MHC class II on DC-1 by the treatment of vitamin C. Even though it is in cancer cells, we found that vitamin C regulates the activation of p38MAPK in our previous study (19). Therefore, the changing on the expression of those three molecules on DC-1 by the treatment of inhibitor for p38MAPK, SB203580, prior to the treatment of vitamin C was examined. As shown in Fig. 3A, we found that the expressions of CD80, CD86 and MHC class II on DC-1 were definitely decreased by the pre-treatment of SB203580 (Fig. 3A). We confirmed whether vitamin C could induce the phosphorylation of p38MAPK in DC-1 by immunoblotting. As shown in Fig. 3B, the phosphorylation of p38MAPK was increased by the treatment of 2.5 mM of vitamin C. Taken together, vitamin C up-regulates CD80, CD86 and MHC class II on DC-1 via the activation of p38MAPK.
Vitamin C effectively prevents oxidative damages through the scavenging of oxygen radicals. The physiological vitamin C concentration in human serum is known as 70~85µM (20,21). Serum concentration of vitamin C is determined by the route of administration. According to the report by Sebastian J et al, when 3 grams of vitamin C is administered via intravenous injection, concentration of vitamin C in serum is reached at 1,700µM, but administered via oral route; it is reached just 220µM (22). Even though vitamin C used in our experiment is 1 and 2.5 mM and it is much higher than its physiological concentration, serum concentration could be increased by the administration via intravenous injection of vitamin C. When we consider that most of recent trials of anti-cancer therapy by vitamin C is done by intravenous injection, it seems that the combination approach of vitamin C and chemotherapy or radiotherapy will give us the new insight of cancer therapy.
It is reported that the relatively high doses of vitamin C (more than 5 mM) induce the apoptosis on tumor cells. When B16F10 melanoma cells were exposed to vitamin C, vitamin C induces the decrease of mitochondrial membrane potential followed by the release of cytochrome C (23). In addition, it inhibited the uptake of iron, an essential factor for the survival of tumor cells, through the down-regulation of CD71 (transferrin receptor) expression (24). The relatively low doses of vitamin C (less than 1 mM) inhibit proliferation of tumor cells via the regulation of Chk2-p53-p21Waf1/Cip1 pathway and the expression of receptor for growth factor, such as insulin-like growth factor (IGF)-1 (25,26). Moreover, vitamin C could regulate angiogenic process of tumor through the down-regulation of vascular endothelial growth factor (VEGF) production. In this process, the expression of cyclooxygenase-2 (COX-2), a well-known pro-inflammatory mediator, was also down-regulated by vitamin C (27). Even though there are many reports regarding the direct role of vitamin C as an anti-tumor agent, the specific action of vitamin C as an immune regulator is largely unknown. We have previously reported that the surface expression of CD95 (Fas) on human stomach cancer cell line, SNU-1 was induced by the treatment of vitamin C (28). It suggests that the immune susceptibility of tumor could be increased by vitamin C. Since dendritic cell is the most potent APC to generate tumor specific T cells, we investigated the role of vitamin C on the maturation of dendritic cell in the present study.
In general, the expression of co-stimulatory molecules, CD80 and CD86, on DCs are known as a crucial secondary signal for the generation of effector T cells. Therefore, the up-regulation of CD80 and CD86 implies the enhancement of activity of DCs. According to the report by Iijima N et al., CD80 and CD86 expression of DCs was up-regulated by the treatment of tumor necrosis factor (TNF)-α (29). It is also reported that concanavalin A (Con A) and lipopolysaccharide (LPS) increases CD80 and CD86 expression on DCs at inflamed sites (30,31). Moreover, it is increased on monocytes in the patients with toxoplasma gondii infections to enhance proliferation of resting T cells in response to parasite-infected cells (32). It suggests that CD80 and CD86 play a crucial role in the initiation and maintenance of an immune response. To the point of this view, constitutively enhanced expressions of CD80 and CD86 by the treatment of vitamin C shown in the present study have the beneficial effect of the maintenance of immune responses. However, the expression of CD80 and CD86 was not up-regulated by the treatment of two of the most potent anti-oxidant, N-acetyl-l-cysteine (NAC) and reduced glutathione (GSH) (29). It seems that it is because of the different properties as an anti-oxidant between vitamin C and NAC and GSH. Vitamin C could be rapidly converted to its oxidized form, dehydroascorbate (DHA). In turn, DHA could be also rapidly converted to its reduced form, ascrobate (vitamin C). In contrast, NAC and GSH did not have redox cycle like vitamin C, even though they are more potent than vitamin C. Moreover, the reduction of glutathione disulfide (GSSG) to GSH is dependent on the action of vitamin C as an electron donor (33). Therefore, the different roles of anti-oxidants on the induction of CD80 and CD86 on DCs should be further investigated.
We have previously reported that vitamin C shows its activity through the regulation of p38MAPK and P42/44MAPK (19,25,27). In the present study, vitamin C regulates the activation of p38MAPK on DC-1. In addition, we found that vitamin C also modulates the activation of phsphoinositide-3 kinase (PI3K)/Akt in hepatocyte (our unpublished data). It might be that vitamin C shows its action after being transported into the cells through specific channel proteins. The reduced form of vitamin C, ascorbate is transported by sodium-dependent vitamin C transporter (SVCT)-1/2, and the oxidized form of vitamin C, dehydroascorbate (DHA) is transported by glucose transporter (GLUT) (34-36). That is to say, the phosphorylation of kinases by vitamin C is not mediated by receptor associated adaptor proteins, since SVCT-1/2 and GLUT are not receptor proteins, but channel proteins. Since we have shown that the intracellular ROS level was not decreased, when DC-1 cells were cultured medium without sodium, it seems that the activation of kinases is dependent on the activity of SVCT-1/2. To examine the mechanisms and its related molecules on the regulation of SVCT-1/2 activity on immune cells, including dendritic cells, we are now investigating with L-gulono-γ-lactone oxidase (Gulo) knock-out mice, which cannot synthesize vitamin C like a human (37).
Figures and Tables
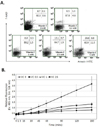 | Figure 1Vitamin C acts as an effective anti-oxidant on DC-1. (A) DC-1 cells were cultured in the presence of 0, 0.5, 1, 2.5 and 5 mM of vitamin C for 24 hr. And the cell viability was examined by flow cytometric analysis, after staining with Annexin V-FITC and 7-AAD. (B) Cells were cultured in the presence of 0, 0.5, 1, and 2.5 mM of vitamin C and 50µM of 2',7'-dichlorofluorescein diacetate (DCFH-DA) at 37℃. And then the changing on intracellular ROS levels were analyzed with a Cytofluor 2350 plate reader with excitation at 485 nm and emission at 525 nm. p-value between 0 mM of vitamin C and 0.5, 1 and 2.5 mM was less than 0.001. |
 | Figure 2Vitamin C up-regulates the expression of CD80, CD86 and MHC class II on DC-1. DC-1 cells were cultured in the presence of 1 and 2.5 mM of vitamin C for 24 hr. And then the expression of CD80, CD86 and MHC class II was examined by flow cytometric analysis, after staining with FITC conjugated anti-CD80, CD86, and MHC class II, as described in Materials and Methods. |
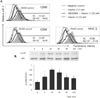 | Figure 3Vitamin C up-regulates CD80, CD86 and MHC class II expression on DC-1 via the activation of p38 MAPK. (A) DC-1 cells were pre-treated with 10µM of SB203580, a specific inhibitor for p38MAPK, for 30 min. And then washed with PBS twice and cultured for another 24 hrs in the absence or presence of 2.5 mM of vitamin C. And then the expression of CD80, CD86 and MHC class II was examined by flow cytometric analysis, after staining with FITC conjugated anti-CD80, CD86, and MHC class II, as described in Materials and Methods. (B) DC-1 cells were pre-treated with 2.5 mM of vitamin C for indicated time. And then the phosphorylation of p38MAPK in DC1 upon the treatment of vitamin C was examined by western blot analysis. The density of each band was measured by densitometry and compared with that of p38 MAPK. Values were represented as the ratio of phosphorylated p38MAPK to unphosphorylated p38MAPK. *p<0.05 vs. non-treated control of tree independent experiments. |
ACKNOWLEDGEMENTS
This work was supported by the grants from the National R&D Program for Cancer Control (Grant #1020030), Ministry of Health, Welfare & Family Affairs to Jae Seung Kang.
References
1. Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004. 27:101–106.

2. Hartel C, Puzik A, Gopel W, Temming P, Bucsky P, Schultz C. Immunomodulatory effect of vitamin C on intracytoplasmic cytokine production in neonatal cord blood cells. Neonatology. 2007. 91:54–60.

3. Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem. 1990. 265:2584–2587.

4. May JM, Huang J, Qu ZC. Macrophage uptake and recycling of ascorbic acid: response to activation by lipopolysaccharide. Free Radic Biol Med. 2005. 39:1449–1459.

5. Washko P, Rotrosen D, Levine M. Ascorbic acid in human neutrophils. Am J Clin Nutr. 1991. 54(6 Suppl):1221S–1227S.

6. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996. 93:3704–3709.

7. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980. 33:71–76.

8. Anderson R, Smit MJ, Joone GK, Van Staden AM. Vitamin C and cellular immune functions. Protection against hypochlorous acid-mediated inactivation of glyceraldehyde-3-phosphate dehydrogenase and ATP generation in human leukocytes as a possible mechanism of ascorbatemediated immunostimulation. Ann N Y Acad Sci. 1990. 587:34–48.
9. Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. Vitamin C for the treatment of recurrent furunculosis in patients with imparied neutrophil functions. J Infect Dis. 1996. 173:1502–1505.

10. Jacob RA, Kelley DS, Pianalto FS, Swendseid ME, Henning SM, Zhang JZ, Ames BN, Fraga CG, Peters JH. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr. 1991. 54:6 Suppl. 1302S–1309S.

11. Heuser G, Vojdani A. Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: the role of protein kinase-C. Immunopharmacol Immunotoxicol. 1997. 19:291–312.

12. Noh K, Lim H, Moon SK, Kang JS, Lee WJ, Lee D, Hwang YI. Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005. 98:63–72.

13. Kay NE, Holloway DE, Hutton SW, Bone ND, Duane WC. Human T-cell function in experimental ascorbic acid deficiency and spontaneous scurvy. Am J Clin Nutr. 1982. 36:127–130.

14. Lee CW, Wang XD, Chien KL, Ge Z, Rickman BH, Rogers AB, Varro A, Whary MT, Wang TC, Fox JG. Vitamin C supplementation does not protect L-gulono-gamma-lactone oxidase-deficient mice from Helicobacter pylori-induced gastritis and gastric premalignancy. Int J Cancer. 2008. 122:1068–1076.

15. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.

16. Kumaraguru U, Pack CD, Rouse BT. Toll-like receptor ligand links innate and adaptive immune responses by the production of heat-shock proteins. J Leukoc Biol. 2003. 73:574–583.

17. Asai Y, Makimura Y, Ogawa T. Toll-like receptor 2-mediated dendritic cell activation by a Porphyromonas gingivalis synthetic lipopeptide. J Med Microbiol. 2007. 56:459–465.

18. Gaddis DE, Michalek SM, Katz J. Requirement of TLR4 and CD14 in dendritic cell activation by Hemagglutinin B from Porphyromonas gingivalis. Mol Immunol. 2009. 46:2493–2504.

19. Sinha A, Singh A, Satchidanandam V, Natarajan K. Impaired generation of reactive oxygen species during differentiation of dendritic cells (DCs) by Mycobacterium tuberculosis secretory antigen (MTSA) and subsequent activation of MTSA-DCs by mycobacteria results in increased intracellular survival. J Immunol. 2006. 177:468–478.

20. Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/- mouse mutants. J Immunol. 2010. 184:582–590.

21. Cho D, Hahm E, Kang JS, Kim YI, Yang Y, Park JH, Kim D, Kim S, Kim YS, Hur D, Park H, Pang S, Hwang YI, Lee WJ. Vitamin C downregulates interleukin-18 production by increasing reactive oxygen intermediate and mitogen-activated protein kinase signalling in B16F10 murine melanoma cells. Melanoma Res. 2003. 13:549–554.

22. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996. 93:3704–3709.

23. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001. 98:9842–9846.

24. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004. 140:533–537.

25. Kang JS, Cho D, Kim YI, Hahm E, Yang Y, Kim D, Hur D, Park H, Bang S, Hwang YI, Lee WJ. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003. 52:693–698.

26. Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005. 204:192–197.

27. Hahm E, Jin DH, Kang JS, Kim YI, Hong SW, Lee SK, Kim HN, Jung da J, Kim JE, Shin DH, Hwang YI, Kim YS, Hur DY, Yang Y, Cho D, Lee MS, Lee WJ. The molecular mechanisms of vitamin C on cell cycle regulation in B16F10 murine melanoma. J Cell Biochem. 2007. 102:1002–1010.

28. Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008. 216:180–188.

29. Kim HN, Kim H, Kong JM, Bae S, Kim YS, Lee N, Cho BJ, Lee SK, Kim HR, Hwang YI, Kang JS, Lee WJ. Vitamin C down-regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J Cell Biochem. 2011. 112:894–901.

30. Yu Y, Bae S, Kim H, Kim Y, Chu NB, Chu NK, Kang JS, Lee WJ. The Anti-tumor Activity of Vitamin C via the Increase of Fas (CD95) and MHC I Expression on Human Stomach Cancer Cell Line, SNU1. Immune Netw. 2011. 11:210–215.

31. Iijima N, Yanagawa Y, Iwabuchi K, Onoé K. Selective regulation of CD40 expression in murine dendritic cells by thiol antioxidants. Immunology. 2003. 110:197–205.

32. Tomiyama C, Watanabe H, Izutsu Y, Watanabe M, Abo T. Suppressive role of hepatic dendritic cells in concanavalin A-induced hepatitis. Clin Exp Immunol. 2011. 166:258–268.

33. Chen KD, Hsu LW, Goto S, Yeh CW, Nakano T, Lai CY, Chang YC, Hou CH, Wang CC, Cheng YF, Chiu KW, Lin CC, Chen CL. Adaptor protein Shc acts as an immune-regulator for the LPS-stimulated maturation of bone marrow-derived dendritic cells. BMC Immunol. 2011. 12:32.

34. Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007. 274:1–22.
35. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999. 399:70–75.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download