Abstract
Background
Toll-like receptors (TLRs) have been extensively studied in recent years. However, functions of these molecules in murine B cell biology are largely unknown. A TLR4 stimulant, LPS is well known as a powerful polyclonal activator for murine B cells.
Methods
In this study, we explored the effect of a murine TLR9 stimulant, M6-395 (a synthetic CpG ODNs) on B cell proliferation and Ig production.
Results
First, M6-395 was much more potent than LPS in augmenting B cell proliferation. As for Ig expression, M6-395 facilitated the expression of both TGF-β1-induced germ line transcript α (GLTα) and IL-4-induced GLTγ1 as levels as those by LPS and Pam3CSK4 (TLR1/2 agonist) : a certain Ig GLT expression is regarded as an indicative of the corresponding isotype switching recombination. However, IgA and IgG1 secretion patterns were quite different--these Ig isotype secretions by M6-395 were much less than those by LPS and Pam3CSK4. Moreover, the increase of IgA and IgG1 production by LPS and Pam3CSK4 was virtually abrogated by M6-395. The same was true for the secretion of IgG3. We found that this unexpected phenomena provoked by M6-395 is attributed, at least in part, to its excessive mitogenic nature.
All living organisms are exposed constantly to microorganisms that are present in the environment and need to cope with invasion of these organisms into the body. Toll-like receptors (TLRs) are of interest to immunologists because of their front-line role in the initiation of innate immunity against invading pathogens (1). Engagement of TLRs by the cognate antigen triggers clonal expansion of the lymphocyte and further production of antigen-specific antibodies. TLR1 and TLR2 sense bacterial triacylated lipopeptides, while TLR4 and TLR9 recognizes LPS and CpG-containing DNA motifs, respectively (1). Among these, TLR9 is expressed in the endoplasmic reticulum and recruited to endosomal/lysosomal compartments once stimulated (2).
Recent studies reveal that TLR stimulation on B cells can control isotype switching. TLR and MyD88 are required for class switching to the pathogenetic IgG2a and IgG2b isotypes but not for development of IgM autoantibodies (3). TLR9 can regulate isotype switching to IgG2a directly by interacting with B cells rather than indirectly by inducing Th1 responses (4). Furthermore, CpG, but not LPS, upregulates T-bet expression in B cells and induces IgG2a whereas it decreases IgG1 and IgE production (5). Other than these studies, the exact role of TLR-9 in Ig synthesis has not been thoroughly investigated yet. Nevertheless, TLR9 ligands would be regarded as the vaccine adjuvant since it enables B cells to stimulate Ig syntheses.
Recently, it has been demonstrated that a synthetic CpG oligodeoxynucleotide (ODN) M6-395 function as TLR9-specific ligands, making them useful in the study of TLR9 functionality and signaling in mice (6). In the present study, we investigated the functions of M6-395 in the context of Ig synthesis paralleling with LPS and Pam3CSK4 (TLR1/2 ligand). In essence, we found that M6-395 can act as a B cell polyclonal activator and that it is much more mitogenic than LPS and Pam3CSK4.
BALB/C mice were purchased from Daehan Biolink Co. (Seoul, Korea) and maintained on an 8:16 h light:dark cycle in an animal environmental control chamber (Myung Jin Inst. Co., Seoul, Korea). Animal care was in accordance with the institutional guidelines of Kangwon National University. Eight- to twelve-week-old female mice were used in this study.
TGF-β1 and IL-4 were purchased from R&D Systems (Minneapolis, MN, USA). 2,2'-Azinobis-(3-ethylbenzthiazoline sulphonic acid) (ABTS) and LPS (Escherichia coli 0111:B4) was from Sigma Chemical Co. (St. Louis, MO, USA). Pam3CSK4 was from Invivogen (San Diego, CA, USA). TRIZOL reagent and CFSE kit were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). The antibodies used in ELISA were purchased from Southern Biotechnology (Birmingham, AL).
Mouse spleen B cell population was prepared as described before (7).
A total of 2×106 cells/well were cultured in flat-bottomed, 24-well tissue culture plates (SPL, Korea) in a volume of 2 ml complete medium or a total of 2×105 cells/well were cultured in flat-bottomed, 96-well tissue culture plates in a volume of 200µl complete medium with added stimulants.
Produced antibodies were detected by using a modified ELISA (8). Isotype specificity was confirmed by assaying isotype-specific antibodies against a panel of myeloma proteins of the IgG isotypes, IgA and IgM. The assay was sensitive to antibody concentrations of 1 to 10 ng/ml.
RNA preparation, reverse transcription, and PCR were performed as described previously (7). PCR primers were synthesized by Bioneer Corp. (Seoul, Korea): TLR1 sense, 5'-GTT GTC ACT GAT GTC TTC AGC-3' and antisense, 5'-GCT GTA CCT TAG AGA ATT CTG-3'; TLR2 sense, 5'-TGG TGT CTG GAG TCT GCT GTG-3' and antisense, 5'-CGC TCC GTA CGA AGT TCT CAG-3'; TLR4 sense, 5'-CAG TGG TCA GTG TGA TTG TGG-3' and antisense, 5'-TTC CTG GAT GAT GTT GGC AGC-3'; TLR9 sense, 5'-CCC TCC TGG TAG AGG CTG C-3' and antisense, 5'-TCT TGT AGT AGC AGT TCC CG-3'; GLTα sense, 5'-CAA GAA GGA GAA GGT GAT TCA G-3' and antisense, 5'-GAG CTG GTG GGA GTG TCA GTG-3'; GLTγ1 sense, 5'-CAG CCT GGT GTC AAC TG-3' and antisense, 5'-CTG TAC ATA TGC AAG GCT-3'; AID sense, 5'-TGC TAC GTG GTG AAG AGG AG-3' and antisense 5'-TCC CAG TCT GAG ATG TAG CG-3'; β-actin sense, 5'-CAT GTT TGA GAC CTT CAA CAC CCC-3' and antisense, 5'-GCC ATC TCC TGC TCG AAG TCT AG-3'. PCR reactions for β-actin were performed in parallel in order to normalize cDNA concentrations within each set of samples. PCR products were separated on a 2% agarose gel and photographed. Band intensities were quantified using Scion Image software (Scion Corp., Frederick, MD, USA).
Toll-like receptor plays an important role in the biology of B cells (9). First of all, we found that TLR1, TLR2, TLR4, and TLR9 were expressed in mouse B cells at the transcriptional level (Fig. 1A). LPS, a TLR4 stimulant, is a powerful mitogen for murine B cells. Therein, we examined the mitogenic effect of M6-395 on the viability of mouse B cells paralleling with LPS. LPS, at the concentration of 12.5µg/ml, markedly increased B cell viability up to day 4 (Fig. 1B). Herein, 100 nM of M6-395 was comparable to 12.5µg/ml of LPS in the increment of cell viability. In fact, the former appears to be more mitogenic against mouse B cells than the latter since it took 3 days for M6-395 and 4 days for LPS to reach the maximal viability. We further characterized the mitogenic property of M6-395 by CFSE assay. In this, Pam3CSK4 (a TLR1/2 stimulant) was also included since it was expressed in B cells (Fig. 1) and thus expected to be stimulatory for B cells. As shown in Fig. 1C, M6-395 stimulated B cell proliferation much better than LPS or Pam3CSK4. This finding was consistent with the earlier report that CpG promotes cell cycle entry and cell survival (10).
Since M6-395 strongly increased B cell proliferation, it was important to explore whether it affects Ig production by B cells. As shown in Fig. 2A, we found that M6-395 facilitated secretion of IgM, IgG subclasses, and IgA. However, the amounts of each Ig in the presence of M6-395 were less than those in the presence of LPS or Pam3 CSK4. Subsequently, we examined if M6-395 can regulate expression of activation-induced cytidine deaminase (AID), which is a critical enzyme for general class switching recombination (CSR) from IgM to other Ig isotypes (11,12). As shown in Fig. 2B, M6-395, like LPS and Pam3CSK4, clearly induced AID expression at the transcriptional level. These results indicate that M6-395 enhances IgG and IgA secretion, at least in part, through AID-mediated Ig CSR.
Since M6-395 can enhance Ig CSR as shown in Fig. 2A, it was necessary to determine whether M6-395 supports a certain cytokine-specific Ig CSR. TGF-β1 and IL-4 are potent IgA and IgG1 switching factors, respectively (13,14). Given that the expression of a particular Ig germline transcript (GLT) is considered to be an indicative of such Ig CSR (15), we examined the effects of M6-395 on TGFβ1-inducible GLTα and IL-4-inducible GLTγ1 expression. M6-395, like LPS and Pam3CSK4, well supported both cytokine-induced GLT expression, indicating that M6-395 has an activity to induce Ig CSR (Fig. 3A). Interestingly, the increasing GLTα and GLTγ1 expression by M6-395 was not further augmented in the presence of additional LPS or Pam3CSK4, suggesting that the three reagents may share common pathway toward general Ig CSR event.
On the other hand, both TGFβ1-induced IgA secretion and IL-4-induced IgG1 secretion were apparently increased by M6-395 but the amounts of each isotype were much less than those by LPS and Pam3CSK4 (Fig. 3B). Moreover, the striking increase of IgA and IgG1 by either LPS or Pam3CSK4 were virtually abolished in the presence of additional M6-395. These are totally unexpected results, which are also sharply contrasted to those seen in expression patterns of GLTα and GLTγ1. This inhibitory effect of M6-395 was more dramatic in IgG3 secretion by LPS and Pam3CSK4 (Fig. 3B).
We speculated that the inhibitory function of M6-395 in LPS- and Pam3CSK4-stimulated Ig production is at least in part owing to an inherent anti-proliferative activity of M6-395. Therein, the present study tested the combined effect of LPS and M6-395 on the proliferation of splenic B cells. As shown in Fig. 4, proliferation of B cells treated with LPS plus M6-395 was greater than that of LPS and M6-395 each alone. Taken together, an excess cell proliferation-promoting nature of M6-395 is certainly unfavorable toward B cell differentiation into Ig synthesis.
In the present study, we found that M6-395, a newly identified TLR9 stimulant, is a powerful polyclonal activator for mouse B cells. Surprisingly, this property of M6-395 is even greater than that of LPS, a well-known B cell polyclonal activator. Nevertheless, we also realized that this strong proliferation enhancing activity of M6-395 is detrimental for the ultimate Ig synthesis though Ig CSR event is little affected. Taken together, the results from the present study indicate that M6-395 could be utilized for a direct B cell adjuvant for vaccination. To optimize this adjuvant activity of M6-395, one should consider the dynamics between proliferation and differentiation of B cells initiated by this chemical.
Figures and Tables
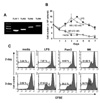 | Figure 1Effect of M6-395 on the viability and proliferation of mouse spleen B cells. (A) TLR expression was detected by RT-PCR in BALB/C mouse spleen B cells. (B) Mouse spleen B cells were cultured with LPS (12.5µg/ml) and M6-395 (20 nM, 100 nM). Cell viability was assessed using trypan blue staining. (C) Mouse spleen B cells were cultured with LPS (12.5µg/ml), Pam3CSK4 (0.5µg/ml) and M6-395 (100 nM). B cell proliferation was assessed after 2 and 3 days analyzing the dilution of CFSE. |
 | Figure 2Effect of M6-395 on Igs production and AID expression. Mouse spleen B cells were cultured with LPS (12.5µg/ml), Pam3CSK4 (0.5µg/ml) and M6-395 (100 nM) for 7 days or 48 h. (A) Supernatants were collected, and Igs productions were determined by isotype-specific ELISA. Data are means of triplicate samples±SEM. (B) Levels of AID and β-actin were measured by RT-PCR after 48 h. |
 | Figure 3Effect of TGF-β1, IL-4 on expression of M6-395-stimulated germline transcripts and Ig production. Mouse spleen B cells were cultured with LPS (12.5µg/ml), Pam3CSK4 (0.5µg/ml) and/or M6-395 (100 nM) and stimulated with TGF-β1 (0.2 ng/ml) or IL-4 (10 ng/ml) for 48 h or 7 days. (A) Levels of germline transcript (GLT) α, GLTγ1, and β-actin were measured by RT-PCR. (B) Supernatants were collected and Igs productions were determined by isotype-specific ELISA. Data are means of triplicate samples±SEM. |
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0017247 and The Regional Core Research Program/Medical & Bio-Material Research Center) and by the 2nd stage of the Brain Korea 21 program.
References
1. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007. 7:353–364.
2. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004. 5:190–198.

3. Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006. 203:553–561.

4. Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007. 178:2415–2420.

5. Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003. 4:687–693.

6. Choi SS, Chung E, Jung YJ. Newly identified CpG ODNs, M5-30 and M6-395, stimulate mouse immune cells to secrete TNF-alpha and enhance Th1-mediated immunity. J Microbiol. 2010. 48:512–517.

7. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001. 31:1706–1715.

8. Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987. 139:2669–2674.
9. Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J Immunol. 2005. 174:6639–6647.

10. Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998. 160:5898–5906.
11. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000. 102:553–563.

12. Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000. 102:565–575.

13. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990. 145:3773–3778.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download