Abstract
Respiratory syncytial virus (RSV) and influenza virus are the most significant pathogens causing respiratory tract diseases. Composite vaccines are useful in reducing the number of vaccination and confer protection against multiple infectious agents. In this study, we generated fusion of RSV G protein core fragment (amino acid residues 131 to 230) and influenza HA1 globular head domain (amino acid residues 62 to 284) as a dual vaccine candidate. This fusion protein, Gcf-HA1, was bacterially expressed, purified by metal resin affinity chromatography, and refolded in PBS. BALB/c mice were intranasally immunized with Gcf-HA1 in combination with a mucosal adjuvant, cholera toxin (CT). Both serum IgG and mucosal IgA responses specific to Gcf and HA1 were significantly increased in Gcf-HA1/CT-vaccinated mice. To determine the protective efficacy of Gcf-HA1/CT vaccine, immunized mice were challenged with RSV (A2 strain) or influenza virus (A/PR/8/34). Neither detectable viral replication nor pathology was observed in the lungs of the immune mice. These results demonstrate that immunity induced by intranasal Gcf-HA1/CT immunization confers complete protection against both RSV and homologous influenza virus infection, suggesting our Gcf-HA1 vaccine candidate could be further developed as a dual subunit vaccine against RSV and influenza virus.
Since the respiratory syncytial virus (RSV) was initially isolated from chimpanzee in 1956, it has been identified as an important cause of lower respiratory tract disease in infants, children, and elderly worldwide. In the late 1960s, formalin inactivated-RSV (FI-RSV) vaccine was evaluated in clinical trials in infants and children aged 2 months to 9 years (1). The FI-RSV vaccine appeared at the time to be moderately immunogenic and well tolerated (2). However, upon subsequent natural RSV infection, FI-RSV-vaccinated infants and children experienced enhanced pulmonary disease characterized by exaggerated CD4+ T cell responses and pulmonary eosinophilia (3-5). Following the failure of the FI-RSV vaccine, there have been long efforts to develop a working vaccine for RSV. However, there is no safe and effective RSV vaccine available yet. RSV glycoprotein (G protein) is an attractive target for vaccine development since it is highly immunogenic and is one of the most important targets of neutralizing antibodies (6). The central core region of the G protein is relatively well-conserved between A and B subtypes of RSV, and immunization with recombinant adenovirus-based vaccine expressing this region derived from RSV A2 strain has shown to confer complete protection against RSV A subtype infections and nearly complete protection against RSV B subtype infection (7,8).
Influenza virus is another important respiratory pathogen and a major cause of morbidity and mortality around world. It has been estimated that annual epidemics result in three to five million cases of severe illnesses and deaths in high risk populations. The influenza virus surface glycoprotein, Hemagglutinin (HA), is the most targeted component of influenza vaccines currently being used. Immune responses to HA lead to protection against viral infection mediated mainly by the induction of antibodies that neutralize the infectivity of influenza virus (9).
In this study, we generated a fusion protein, Gcf-HA1, consisting of central conserved RSV G protein fragment and HA1 globular head region of influenza PR8 virus as a dual subunit vaccine against RSV and influenza virus infections. We evaluated the immunogenicity and protective efficacy of Gcf-HA1 vaccination by mucosal delivery with cholera toxin as an adjuvant.
The coding sequence of RSV A2 G protein from amino acid residues 131 to 230 and influenza PR8 HA protein from amino acid residues 62 to 284 were amplified from cDNA by PCR. The coding sequence of G was cloned into Xho I and Hind III sites and the coding sequence of HA was cloned into Hind III and EcoR I sites of pET-21d(+) vector (Novagen). The constructed plasmid was transformed into E. coli BL21(DE3) competent cells (Novagen) and overexpression was induced by adding 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG, Takara, Shiga, Japan) at 37℃ for 4 h. Bacterial cells were harvested, resuspended in PBS, and sonicated. Soluble and insoluble fractions were separated by centrifugation at 27,000 g for 30 min and insoluble fraction was resuspended in a binding buffer (20 mM KPO4, 0.5 M NaCl, 20 mM imidazole, 6 M urea, pH 8.0). Again, soluble and insoluble proteins were separated by centrifugation at 27,000 g for 30 min. Then, the supernatants were subjected to affinity chromatography using HisTrap column (GE Healthcare). After washing with the binding buffer, loaded proteins were eluted with elution buffer (20 mM KPO4, 500 mM NaCl, 500 mM imidazole, 6 M urea, pH 7.4). Eluted protein fractions were collected and buffer-exchanged with PBS by dialysis. The purified Gcf-HA1 protein fractions were treated with 1% Triton X-114 to remove endotoxins at 4℃ for 30 min, followed by incubation at 37℃ for 20 min. The phase containing endotoxin was separated by centrifugation. This cycle was repeated five times. Then, the protein was incubated with SM-2 beads (Bio-Rad, Hercules, CA) at 4℃ for 2 h to remove residual Triton X-114 and filtered through spin-X column (Costar, Washington, DC). The purified protein samples were subjected to 12% SDS-PAGE for analysis. Purified proteins were stored in aliquots at -80℃ until use.
The HEp-2 cells were propagated in MEM (Life Technologies, Gaithersburg, MD) supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 20 mM HEPES, nonessential amino acids, penicillin, and streptomycin. The RSV A2 strain was grown by infecting mono-layered HEp-2 cells with MOI of 0.01. Cells were harvested when the infected HEp-2 cell showed maximal cytopathic effect, sonicated for 1 min, and then centrifuged at 300 g for 10 min. The supernatants were collected and centrifuged at 75,000 g for 1 h. The virus pellets were resuspended with serum-free MEM by using 25-gauge needle and sonication. The final titer was determined by standard plaque assay. The influenza PR8 virus was amplified in 11-day-old embryonated chicken eggs for 3 days at 37℃. Allantoic fluids were harvested and filtered through 0.25µm filter. Virus stock was titrated and stored at -80℃ until use.
Female BALB/c mice (five weeks of age) were purchased from Charles River Laboratories (Yokohama, Japan) and kept under specific pathogen-free conditions during the experiments. For intranasal (i.n.) immunization, mice were anesthetized by isoflurane inhalation and injected with 30µg Gcf-HA1 protein with 2µg CT in a volume of 50µl through two nostrils. For boost immunization, the mice were inoculated with the same amount of Gcf-HA1/CT two weeks after the primary immunization. For virus challenge experiments, the groups of mice were infected intranasally with 1×106 PFU of RSV A2 strain or 10 LD50 of influenza PR8 virus three weeks after the boost immunization.
Antibody titers of antigen-specific IgG in the sera and antigen-specific IgA in BAL fluids were measured by direct ELISA. Briefly, 96-well plates were coated with 50 ng of purified RSV Gcf (10) or 200 ng of formalin inactivated influenza PR8 virus diluted in 100µl of PBS overnight, and blocked with PBS containing 1% skim milk for 2 hr. The plates were washed with PBS containing 0.05% Tween-20 five times and sera and BAL fluids in serial dilution were added and incubated for 2 hr. The plates were then washed with PBS containing 0.05% Tween-20 five times and incubated for 1 hr with HRP-conjugated goat anti-mouse IgG (BD Pharmingen) or IgA (Zymed Laboratories, San Francisco, CA) secondary antibody. The plates were washed three times, developed with tetramethylbenzidine peroxidase substrate (KPL, Gaithersburg, MD), stopped with 1 M H3PO4, and analyzed at 450 nm by a Thermo ELISA plate reader.
Five days after virus challenge, mice were sacrificed and bronchoalveloar lavage (BAL) fluids were collected by flushing the lung airways with 0.8 ml of PBS/EDTA. The collected BAL cells and supernatants were used in measuring leukocytes and secretory IgA titers, respectively. Briefly, BAL cells were incubated for 15 minutes with CD16/32 blocking antibody, stained with FITC-conjugated anti-Gr-1(RB6-8C5), PE-conjugated anti-siglec-F(E50-2440), PE-Cy5-conjugated anti-CD11c(N418), and APC-conjugated anti-CD45(H130). After staining, cells were acquired using FACSCalibur flow cytometer (BD Bioscience, San Diego, CA). The frequency of infiltrated neutrophils and eosinophils in the BAL was measured via flow cytometry using Gr-1-, Siglec-F-, CD45- and CD 11c-specific antibodies.
Five days after RSV or influenza virus challenge, mice were sacrificed and lungs were removed into MEM. The sliced lung tissues were processed through a 70µm cell strainer (BD Labware, Franklin Lakes, NJ). The lung supernatants were collected and RSV titers in the supernatants were measured by standard plaque assay on HEp-2 cell monolayers. The influenza virus titers in the lung tissues were determined by standard plaque assay on MDCK cells. The data are expressed as PFU per gram of lung tissue. The limit of detections for RSV and influenza virus are 100 PFU/g and 200 PFU/g, respectively.
Dual or multiplex vaccines are useful in conferring protection against multiple infectious agents and reducing the number of vaccination. To prepare a dual vaccine against both RSV and influenza infection, a pET-21-based expression plasmid was constructed by fusion of RSV G protein core fragment sequence (amino acid residues 131 to 230) and HA1 globular head domain sequence (residues 62 to 284) from influenza PR8 virus (Fig. 1A). SDS-PAGE analysis showed that ~36 kDa fusion protein of expected size was correctly expressed following IPTG-meidated induction of protein expressions in E. coli, purification by affinity chromatography under denaturing conditions, and subsequent refolding of the protein in PBS (Fig. 1B).
To examine whether mucosal vaccination of Gcf-HA1 fusion protein together with CT as a mucosal adjuvant (11) generates specific humoral responses against two antigens consisting our dual vaccine, BALB/c mice were inoculated twice via i.n. route at days 0 and 14 as shown in Fig. 2A. As a positive control, Gcf/CT was also given twice by i.n. route. We previously reported the immunogenicity of mucosal Gcf/CT vaccine elsewhere (10). Gcf-HA1/CT group showed a little bit of lower levels of Gcf-specific serum IgG responses than the Gcf/CT group, when determined by ELISA (Fig. 2B). This group of mice also showed significant HA-specific serum IgG antibody response (Fig. 2C), while Gcf/CT-immunized mice did not produce any significant HA-specific antibody response (data not shown). These results indicate that mucosal Gcf-HA1/CT vaccination successfully elicits serum antibody responses to both RSV G and influenza HA antigens.
Secretory IgA has been shown to be one of the important immune correlates of protection against RSV infection (7,12). In order to evaluate the efficacy of Gcf-HA1 vaccination in inducing Gcf-specific mucosal IgA response, the immune mice were challenged with RSV A2 strain and ELISA was performed with the BAL fluid collected at day 5 after challenge. The results showed that mucosal Gcf-HA1/CT vaccination via intranasal route elicited significant Gcf-specific IgA titers, which are comparable to the titers of Gcf/CT group (Fig. 3A). In order to determine HA-specific mucosal IgA response, BAL was collected from mice that were vaccinated with Gcf-HA1/CT and challenged with influenza PR8 virus at day 5 post-challenge. ELISA was performed with the collected BAL using formalin-inactivated PR8 as the coating antigen. The results demonstrated that the levels of HA-specific mucosal IgA antibody were significantly elevated in Gcf-HA1/CT-vaccinated mice compared to mock-vaccinated mice (Fig. 3B). Together, these results indicate that mucosal Gcf-HA1/CT vaccination effectively induces antigen-specific secretory IgA responses in the respiratory mucosa as well as serum antibody responses.
To evaluate whether mucosal Gcf-HA1 vaccination induces protective immunity against RSV infection, vaccinated mice were challenged with RSV A2 strain, and plaque assay was performed with the lung homogenates harvested at day 5 post-challenge. No detectable RSV replication was observed in the lungs of mice vaccinated with Gcf-HA1/CT, while high levels of RSV replication were detected in the lungs of mice vaccinated with PBS (Fig. 4A). These results indicate that intranasal Gcf-HA1 vaccination provides complete protection against RSV infection.
Exaggerated Th2 CD4+ T cells responses which cause pulmonary eosinophilia is one of the characteristics that define the vaccine-enhanced RSV diseases (13). In order to characterize the immunopathologic potential of Gcf-HA1/CT vaccination, the profiles of lung-infiltrated leukocytes in the BAL fluid were determined following immunization and RSV challenge. The results showed that there were no significant differences in the frequency of recruited neutrophils and eosinophils between Gcf-HA1-vaccinated and mock-vaccinated groups (Fig. 4B). It is noteworthy that the numbers of eosinophils recruited to the lungs after RSV challenge were low, suggesting that mucosal Gcf-HA1/CT vaccination might not induce vaccine-enhanced eosinophilia.
To evaluate the protective efficacy of Gcf-HA1/CT vaccination against influenza virus infection, the immune mice were challenged with 10 LD50 of PR8 virus and viral titers in the lungs were measured by standard plaque assay on MDCK cells. Lungs of Gcf-HA1/CT-immunized mice exhibited no detectable level of influenza virus replication (Fig. 5A), indicating that Gcf-HA1/CT vaccination provides complete protection against influenza virus infection. We also evaluated morbidity by monitoring weight loss in influenza virus-challenged mice. The group of Gcf-HA1/CT-immune mice did not show any significant weight loss until day 10 after the challenge, while 100% of mock-immunized mice succumb to death at day 6 (Fig. 5B). Overall, these results clearly demonstrate that intranasal Gcf-HA1/CT vaccination confers protective immunity against lethal influenza virus infection without any severe morbidity.
In the present study, we constructed and prepared a dual subunit vaccine candidate, Gcf-HA1, containing RSV G protein fragment and HA1 domain of influenza PR8 virus and investigated the immunogenicity and protective immunity generated by mucosal vaccination of CT-adjuvanted Gcf-HA1.
So far, myriad of RSV vaccine candidates have targeted the two viral surface envelope proteins, G and F, due to their ability to induced neutralizing antibody responses, the presence of which correlates with protection against RSV infection. In our study, RSV G fragment containing the central conserved region was used as the vaccine candidate against RSV infection. The RSV G fragment used in our study corresponds to the amino acid residues 131 to 230 in RSV A2 G protein, which includes the 13 residue-long amino acid sequence that are fully conserved between the two known RSV strains A and B as well as cysteine-noose region containing the CX3C chemokine motif (14). Moreover, multiple protective B cell epitopes were identified within this fragment (15) corroborating our approach of using RSV G fragment as vaccine candidate an appropriate strategy in eliciting antibody-mediated protection against RSV infection.
The hemagglutinin is the major glycoprotein and critical viral target structure for the immune defense mechanisms. Also, the influenza virus can escape the anti-viral immunity of the host population through either antigenic drift or antigenic shift. HA is split into the glycoprotein subunits HA1 and HA2. We targeted HA1 containing most of the antigenic epitopes of the HA glycoprotein. HA1 region could be structurally defined by a disulfide bond between two conserved cysteines, and strongly induces specific neutralizing antibody (16,17). In a previous study, it has been shown that HA1 is highly immunogenic and provides a specific neutralizing antibody, resulting in effective protection against a lethal challenge of influenza virus in the mouse model (17,18).
There are several advantages of developing recombinant multiplex vaccines that target multiple pathogens. First, administration composite vaccines can potentially offer protection against multiple pathogens as our results indicate that mice vaccinated with Gcf-HA1 fusion protein were protected against both RSV and influenza virus infections. Moreover, use of multiplex vaccines can practically reduce the number of inoculations and potentially minimize the inconvenience that may otherwise arise from multiple visits to hospitals for the reception of single-pathogen-specific vaccines. Further, mucosal vaccination is a non-invasive and convenient administration method and targets specific mucosal area, which are optimal properties of vaccines against respiratory pathogens such as RSV and influenza virus. Also, the production of recombinant composite vaccines via bacterial expression system may be more cost-effective than the current methods of producing licensed-vaccines for various pathogens. Thus, our study provides strong evidence that Gcf-HA1 could be further developed as a dual vaccine against two important respiratory pathogens.
Figures and Tables
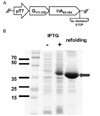 | Figure 1Preparation of Gcf-HA1 subunit vaccine. Expression of RSV G protein core fragment (Gcf: amino acid 131 to 230) fused with influenza HA1 domain (amino acid 76 to 284) was performed in pET-21d-Gcf-HA1-transformed E. coil BL21(DE3). Expressed fusion protein was purified from inclusion body by immobilized metal ion affinity chromatography. Then, Gcf-HA1 fusion protein was refolded in PBS. (A) Sequence structure of pET-21d-Gcf-HA1 expression vector was shown in the diagram. (B) The expression of Gcf-HA1 fusion protein was confirmed by SDS-PAGE of samples from purification steps. |
 | Figure 2Serum antibody responses induced by Gcf-HA1/CT immunization. BALB/c mice were immunized twice on day 0 and 14 by intranasal (i.n) injection of Gcf-HA1 fusion protein in combination with CT. (A) Vaccination and challenge experiment scheme. (B) Serum IgG antibody responses specific for Gcf were measured by ELISA 3 weeks after boosting immunization. (C) Serum IgG antibody titers specific for HA were measured by ELISA 3 weeks after boosting immunization. N.D., not detected. |
 | Figure 3Mucosal antibody responses induced by Gcf-HA1/CT immunization. (A) Mucosal IgA antibody responses specific for Gcf in BAL fluid were measured by ELISA 5 days after RSV challenge. (B) Mucosal IgA antibody titers specific for HA were measured 5 days after influenza PR8 challenge. N.D., not detected. |
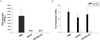 | Figure 4Protective efficacy of Gcf-HA1/CT vaccination against RSV challenge. The immune mice were challenged intranasally with 106 PFU of RSV A2. (A) Lung homogenates were prepared and lung viral titers were measured by standard plaque assay with HEp-2 cells. (B) The numbers of neutrophils and eosinophils in BAL fluids were measured by flow cytometry at day 5 following RSV challenge. N.D., not detected. |
 | Figure 5Protective efficacy of Gcf-HA1/CT vaccination against influenza virus infection. The immune mice were challenged with 10 LD50 of influenza PR8 virus. (A) Lung homogenates were prepared and lung viral titers were measured by standard plaque assay with MDCK cells. (B) Mice were monitored for weight loss each day after challenge. |
ACKNOWLEDGEMENTS
We wish to acknowledge technical support of Dr. Jee-Boong Lee in the completion of this work. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No. 2012-0000952) and by a grant of the TEPIK (Transgovernmental Enterprise for Pandemic Influenza in Korea), part of Korea Healthcare technology R&D Project by Ministry of Health & Welfare, Republic of Korea (Grant No.: A103001).
References
1. Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005. 22:577–587.

2. Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988. 26:1595–1597.

3. Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994. 68:5321–5325.

4. Kim HW, Leikin SL, Arrobio J, Brandt CD, Chanock RM, Parrott RH. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976. 10:75–78.

5. Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996. 70:2852–2860.

6. Collins PL, Purcell RH, London WT, Lawrence LA, Chanock RM, Murphy BR. Evaluation in chimpanzees of vaccinia virus recombinants that express the surface glycoproteins of human respiratory syncytial virus. Vaccine. 1990. 8:164–168.

7. Jang JE, Lee JB, Kim KH, Park SM, Shim BS, Cheon IS, Song MK, Chang J. Evaluation of protective efficacy of respiratory syncytial virus vaccine against A and B subgroup human isolates in Korea. PLoS ONE. 2011. 6:e23797.

8. Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008. 82:2350–2357.

9. Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, Rappuoli R. Influenza vaccine immunology. Immunol Rev. 2011. 239:167–177.

10. Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012. 7:e32226.

11. Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One. 2009. 4:e5190.

12. Kim S, Chang J. Baculovirus-based vaccine displaying respiratory syncytial virus glycoprotein induces protective immunity against RSV infection without vaccine-enhanced disease. Immune Netw. 2012. 12:8–17.

13. Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, Taylor G. Respiratory syncytial virus infection of gene gun vaccinated mice induces Th2-driven pulmonary eosinophilia even in the absence of sensitisation to the fusion (F) or attachment (G) protein. Vaccine. 2000. 19:1038–1046.

14. Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001. 2:732–738.

15. Plotnicky-Gilquin H, Goetsch L, Huss T, Champion T, Beck A, Haeuw JF, Nguyen TN, Bonnefoy JY, Corvaïa N, Power UF. Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J Virol. 1999. 73:5637–5645.

16. Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982. 31:417–427.

17. Khurana S, Larkin C, Verma S, Joshi MB, Fontana J, Steven AC, King LR, Manischewitz J, McCormick W, Gupta RK, Golding H. Recombinant HA1 produced in E. coli forms functional oligomers and generates strain-specific SRID potency antibodies for pandemic influenza vaccines. Vaccine. 2011. 29:5657–5665.

18. Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, Jacobs A, Liu G, Huang Y, Desai P, Maksymiuk G, Takahashi V, Umlauf S, Reiserova L, Bell R, Li H, Zhang Y, McDonald WF, Powell TJ, Tussey L. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS One. 2008. 3:e2257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download