INTRODUCTION
Pancreatic cancer is the fourth commonest cause of cancer-related mortality across the world, with incidence equaling mortality (1). According to the American Cancer Society, for all stages of pancreatic cancers combined, the one-year relative survival rate is 20%, and the five-year rate is 6% (2). These low survival rates are attributable to the fact that fewer than 20% of patients' tumors are confined to the pancreas at the time of diagnosis; in most cases, the malignancy has already progressed to the point where surgical removal is impossible (3). Gemcitabine is a chemotherapeutic drug that has become the standard treatment for advanced diseases after showing superiority over 5-fluorouracil, while the combination of radiation and chemotherapy is still widely used (4). Although gemcitabine-based chemotherapy is typically offered as the standard of care, most patients do not survive longer than 6 months (3). Pancreatic cancer cells that developed gemcitabine resistance would still be suitable targets for immunotherapy (5). Although the combination of radio-, chemo-, and immunetherapy showed improvements in overall survival rates (6), new therapeutic approaches are still needed.
Cytokine-induced killer (CIK) cells are a population of cells derived from human peripheral blood mononuclear cells after ex vivo expansion with interferon-gamma, anti-CD3 antibodies, and interleukin (IL)-2 (7). A majority of CIK cells express T cell receptors, and others express NK cell markers (8). CIK cells have a higher proliferation rate, cytolytic activities and non-MHC-restricted killing of tumor cells in comparison with lymphokine-activated killer (LAK) cells, which are essentially activated by natural killer (NK) cells (9,10). They have been shown to target a variety of tumors and can exert their cytotoxic effects following systemic delivery (11). Although CIK cells might be similar to LAK cells, there were several differences. First, LAK cells are originated from NK cells, but CIK cells are generated from CD8+ T cells (8). Second, LAK cell population mainly include CD3-CD56+ cells, but CIK cells include more than 30% CD3+CD8+CD56+ cells.
Many preclinical and clinical studies showed that CIK cells showed promising anti-tumor effects against various cancers, including hepatoma (12,13), leukemia (14), lung (11,15), ovarian (16), renal (17), and gastric cancer (18). In this study, we examined the antitumor activity of CIK cells against human pancreatic cancer to broaden the clinical applicability of CIK cells. We generated CIK cells from human peripheral blood mononuclear cells, characterized their phenotypes, and evaluated the anti-tumor activity of CIK cells in vitro and in vivo.
MATERIALS AND METHODS
Cells
Human pancreatic cancer AsPC-1 cells and human leukemia K562 cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100µg/ml streptomycin. CIK cells were generated from the PBMCs of volunteers. After the volunteers gave informed consent, 10~20 ml of blood was drawn in evacuated tubes with heparin (Vacutainer, BD Biosciences). The PBMCs were obtained from buffy coats by Ficoll-Hypaque density centrifugation and washed three times with PBS (13). Next, the PBMCs were re-suspended at 1×106 cells/ml in Lymphomedia (Lymphotech) containing 10% fetal bovine serum (Invitrogen) and cultured in the presence of immobilized anti-CD3 antibody (5µg/ml, BD Pharmingen) and recombinant human IL-2 (700 U/ml, R&D Systems) for 5 days. Following this, the cell suspension was further incubated in Lymphomedia containing recombinant human IL-2 only (170 U/ml) for 9 days. Fresh IL-2 and medium were replenished every 2~3 days (16). During the generation period, the cell number was maintained at approximately one million cells per milliliter. The viability of expanded cell populations on day 14 was 85~90%.
Phenotype analysis
Cells were obtained from CIK cultures for phenotype analysis with appropriate monoclonal antibodies, including CD3-FITC as a T cell marker, CD4-FITC as a helper T cell marker, CD8-PE as a cytotoxic T cell marker, and CD56-PE as a natural killer cell marker. One million CIK cells were washed once with PBS containing 1% bovine serum albumin (BSA) and resuspended in 100µl of PBS/BSA buffer. The cells were incubated with various conjugated monoclonal antibodies for 20 min at 4℃, washed twice with PBS, and resuspended in 400µl of PBS. Flow cytometric analysis was performed on a FACSCalibur flow cytometer (BD Biosciences), and the data were analyzed using the WinMDI statistical software (Scripps). Cell viability was examined using the propidium iodide (PI) nuclear staining method. Cells were stained with 1µg/ml of PI and analyzed with FACSCalibur flow cytometer. Cells stained with PI were considered dead (11).
51Cr release assay
The lysis of human cancer cells by CIK cells was measured in a 4-h 51Cr release assay (11,19). Briefly, two million target cells were labeled with 100µCi of sodium chromate (Dupont-NEN) for 4 h at 37℃. The labeled cells were washed three times in PBS and resuspended in 10 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum. The labeled cells were plated in triplicate in round bottom 96-well plates at 1×104 cells/100µl per well. CIK cells were added at specified effector:target (E:T) ratios (1:1, 3:1, 10:1, 30:1 and 100:1) and incubated for 4 h. The supernatant was removed and the radioactivity was measured in a gamma counter. The percentage of specific lysis was calculated according to the following equation: cytotoxicity=[(sample-spontaneous)/( maximum-spontaneous)]×100. Spontaneous release was obtained by incubating target cells in medium alone, whereas maximal release was obtained by treatment with 2% Nonidet P-40 (Sigma).
Nude mouse xenograft assay
Nude mice (6~8 weeks old) were obtained from Charles River and were housed under specific pathogen free conditions according to the guidelines of the Animal Care Committee at the Chungbuk National University. On day 0, nine million AsPC-1 cells in 300µl of PBS were injected subcutaneously into nude mice. Following this, CIK cells were injected intravenously once a week at doses of 1, 3, and 10 million cells per mouse. Adriamycin was injected intravenously once a week at a dose of 2 mg/kg. Tumor volumes were estimated by the formula: length (mm)×width (mm)×height (mm)/2. On day 25, the mice were sacrificed and the tumor mass was weighed. To determine animal toxicity, the body weights of the animals were measured (20).
Statistics
The results represent the mean values of more than three samples and seven mice. Standard deviations (SD) were calculated using the Student's t test and p-values were calculated by ANOVA (GraphPad Software) (21).
RESULTS AND DISCUSSION
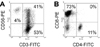
After 14 days, the absolute number of human PBMC cultured in the presence of IL-2 and anti-CD3 antibodies increased by more than 230-fold from 30 million PBMCs to 6,900 million CIK cells. When we examined the phenotypes of the cultured cell population with fluorescence-activated cell sorting analyses, the cell population was composed of 94% CD3+, 43% CD3+CD56+, 41% CD3+CD56+, 11% CD4+, and 73% CD8+ (Fig. 1A and B). Interestingly, most CIK cells were CD8+, but not CD4+. Fresh PBMC usually include less than 5% CD3+CD56+ cells. These results suggested that CIK cells CD3+ T cell population containing CD3+CD56+ cells, as previously reported that CIK cells were generated from CD3+CD8+ precursors (8).
Next, we examined the cytotoxicity of CIK cells under in vitro conditions in a 4-h Cr51 release assay. CIK cells have been demonstrated to have strong cytotoxicity in a non-MHC-restricted manner. At E:T cell ratios of 10:1, 30:1, and 100:1, CIK cells destroyed 22%, 37%, and 51% of AsPC-1 cells, respectively (Fig. 2B). These cells also destroyed 46%, 70%, and 77% of K-562 target cells, respectively (Fig. 2A). We also examined the cytotoxicity of fresh PBMC against these target cells and observed that fresh PBMC destroyed less than 4% of AsPC-1 and K562 cells.
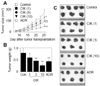
Finally, we evaluated the anti-tumor activity of CIK cells in nude mouse xenograft assays. Preliminary experiments revealed that one hundred million CIK cells did not produce any observable toxicity in nude or SCID mice. Both mice did not exhibit hair ruffling, lowered morbidity, or weight loss (data not shown). Thus, we injected CIK cells intravenously at doses of less than one hundred million cells. Nine million AsPC-1 cells were injected subcutaneously into nude mice and grew to a tumor volume of 251±50 mm3 (n=7) 25 days after implantation (Fig. 3A). CIK cells were injected intravenously at doses of 1, 3, and 10 million cells per mouse and inhibited in vivo tumor growth by 23%, 42%, and 70%, respectively. Adriamycin (ADR), used as a reference drug, strongly inhibited the growth of AsPC-1 tumors.
On day 25, all tumors were isolated from nude mice and weighed, which demonstrated the strong anti-tumor effect of CIK cells against AsPC-1 tumors (Fig. 3B and C). The weight of AsPC-1 tumors increased to 790+/-193 mg 25 days after implantation. CIK cells that were injected intravenously at doses of 3 and 10 million cells per mouse inhibited tumor weight by 42% and 66%, respectively. Adriamycin (ADR), used as a reference drug, inhibited tumor growth by 44%. The body weights of tumor-bearing nude mice were examined to assess the toxicity. Overall, the nude mice used in this study exhibited body weight gains of 120~130%, suggesting that CIK cell therapy does not produce animal toxicity (Fig. 4).
The goal of immune cell-based cancer therapy is to eliminate cancer cells through the transfer of ex vivo expanded and activated immune cells. Immune cells such as dendritic cells (DC) (22), LAK cells (23), natural killer (NK) cells (24), cytotoxic T lymphocytes (CTL) (17), and cytokine-induced killer (CIK) cells (25) have been explored for adoptive immunotherapy of cancer. NK and LAK cell therapy has been hindered by the inherently low anti-tumor activity (9). CTL therapy, in turn, was hindered by the MHC-restricted mechanism, a limited number of tumor-associated antigens, and a low number of tumor-specific CTL (26). In the case of DC therapy, it may be difficult for transplanted DC's to activate effector T cells, which were usually constrained by the severe chemotherapy (11). In contrast, CIK cells had several attractive advantages. First, it is very easy to generate a large number of CIK cells ex vivo and they are readily expandable from PBMC's of cancer patients (27). Second, compared to LAK cells, CIK cells exhibit enhanced cytotoxic activity (28). Third, cytotoxicity is MHC-unrestricted (9,10,29). Fourth, CIK cells are the final effector cells, which are able to directly kill cancer cells (7).
Here, we showed that after 14 days of culturing human PBMC in the presence of IL-2 and anti-CD3 antibodies, the absolute number of cells increased by more than 200-fold. Anti-CD3 antibody has been shown to trigger T cell proliferation (28). As a key cytokine in CIK cell generation, IL-2 increased the cell number, maintained cell viability, and augmented cytotoxicity. From day 6, we incubated cells with only IL-2, resulting in generation of CD3+CD8+CD4 CD56 or CD3+CD8+CD4 CD56+ cells. Although IFN-γ was used in general protocols, we did not use it because our previous observations showed that CIK cells themselves produce high amounts of IFN-γ, which might stimulate the autocrine pathway (11). We simply added fresh medium to the culture bags every 2 or 3 days and split them without removing the old medium (30). Thus it is possible that CIK cell-derived cytokines, especially IFN-γ, accumulated in culture medium.
CIK cells had an effect on various cancers, including hepatoma, leukemia, lung, ovarian, renal, and gastric cancer, in pre-clinical and clinical studies (11-18). Here, we provide additional evidences that CIK cell immunotherapy can be effective in eradicating pancreatic cancers. CIK cells broke down AsPC-1 pancreatic cancer cells in vitro and in vivo. This study suggests that CIK cells are good candidates for cell-based immunotherapy in pancreatic cancer patients.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download