INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease mainly affecting the synovia of diarthrodial joints. The synovium consists of a lining layer of fibroblast-like synoviocytes (FLS) and macrophage-like synoviocytes that overlies the loose connective tissue of the synovial sublining (1,2). During the course of RA, the synovium serves as the site of inflammation where leukocytes infiltrate massively. These cells activate FLS to produce proinflammatory mediators including cytokines, chemokines, and matrix metalloproteases. In addition, activated FLS become hyperplastic and form a pannus tissue that invades articular cartilage and bone. Therefore, FLS act in synergy with leukocyte infiltrates to perpetuate inflammation and to destroy cartilage and bone. On the other hand, the inflammatory milieu also seems to recruit anti-inflammatory cells, since inflamed synovia from patients with RA and animal models contain a higher proportion of regulatory T cells (Tregs) than their peripheral blood or lymph nodes (3-5).
Tregs are a unique population that can suppress the proliferation of conventional T cells and cytokine production by these cells (6). Hence, the activity of Tregs is crucial for suppressing autoimmunity and inflammation. Thymus-derived natural Tregs constitutively express activation markers, such as CD25, CTLA-4 and glucocorticoid-induced TNF receptor family-related gene (GITR), whose expression is also induced in activated conventional T cells; they also express a specific marker named forkhead/winged box p3 (Foxp3). Foxp3 serves as a master transcription factor which programs the development and function of Tregs (7). Foxp3 can be generated in the periphery or in vitro when naïve CD4+ T cells are primed in the presence of TGF-β and IL-2 (8,9). Conversely, it has been shown that a substantial percentage of Tregs stop expressing Foxp3 even in the steady-state (10). These ex-Foxp3 T cells are highly enriched in inflamed tissues in autoimmune conditions, implying that the proinflammatory milieu further causes additional Tregs to stop expressing Foxp3. Indeed, the proinflammatory cytokine IL-6 has been shown to collaborate with IL-1 to downregulate Foxp3 expression in a Stat3-dependent manner (11,12). These ex-Foxp3 T cells lose their regulatory functions and even produce proinflammatory cytokines such as IFN-γ and IL-17, and can be pathogenic when adoptively transferred to normal mice (10). Nevertheless, it remains unclear whether Tregs residing in the inflamed synovium stop expressing Foxp3, and, if so, how this occurs.
To address these questions, we established an in vitro coculture system that can mimic an inflamed synovium housing FLS and Tregs. We found that the FLS signal the Tregs to inhibit their Foxp3 expression, while the Tregs induce the FLS to produce IL-6. Thus we have identified a mechanism by which autoimmune arthritis evolves into a state of chronic, self-amplifying inflammation, despite the normal development of Tregs in central and peripheral lymphoid organs.
MATERIALS AND METHODS
Mice
Six-to-seven-week-old BALB/c mice were purchased from Orient-bio laboratory (Seongnam, Korea). SKG mice breeders were provided by Dr. Shimon Sakaguchi (Osaka University, Osaka, Japan) and their progeny were maintained in the animal facility of Hanyang University. All mice were kept under specific pathogen-free conditions, and all the animal experiments conducted were carried out in accordance with the guidelines provided by Hanyang University.
Establishment of FLS lines
To isolate inflamed synovia we established zymosan-induced arthritis in SKG mice, as described previously (13). Synovial tissues extracted from inflamed ankle joints of the arthritic mice were minced, incubated with 400 Mandl U/ml of Liberase Blendzyme II (Roche) in serum-free DMEM for 1 h at 37℃, and filtered through a nylon mesh. The single cell suspension was washed and cultured in DMEM supplemented with 10% FBS. After culture overnight, non-adherent cells were washed out and the adherent cells were continuously cultured. Passage 2 to 4 FLS cultures were used in these experiments.
Isolation of CD4+CD25+ Tregs, and coculture with FLS
Spleens and lymph nodes were removed from BALB/c mice, and erythrocyte-depleted single-cell suspensions were prepared in RPMI-1640 medium supplemented with 10% FBS. CD4+CD25+ T cells to >95% purity were prepared using MACS columns (Miltenyi Biotec). FLS were seeded in 6-well plates (Corning) at 1×105 cells/well in DMEM supplemented with 10% FBS. After culture overnight, 1×106 Tregs were added to the wells and the mixtures were stimulated with 10 µg/ml anti-CD3 mAb, 1 µg/ml anti-CD28 mAb (BD Pharmingen), and 20 U/ml IL-2 (Peprotech). In some experiments, 10 µg/ml anti-GITR mAb or 10 µg/ml anti-IgG2b mAb (BD Pharmingen) was added to the medium. After culture for 4 days, the cells were harvested for FACS analyses, and the supernatants were collected for cytokine measurement by ELISA.
Flow cytometry
Cells were stained with mAbs and assayed by FACS, as described previously (14). The mAbs used for this study was as follows: anti-CD4-PerCP and anti-CD25-APC were purchased from BD Pharmingen, and anti-Foxp3 FITC, anti-IFNγ-FITC, anti-IL-17-PE, and anti-GITR-PE were from eBioscience. The data were acquired with a BD FACS canto II and analyzed with FACS Diva software.
Cytokine ELISA
The concentration of IL-6 in culture supernatants was measured by quantitative ELISA using R&D Duoset ELISA systems (R&D Systems) according to the manufacturer's instructions.
RT-PCR
The total RNA was isolated from FLS using Trizol reagent (Invitrogen), and reverse-transcribed to generate cDNAs using M-MLV reverse transcriptase (Promega). The gitrl gene was amplified by PCR reactions and normalized to the expression level of the β2 microglobulin (β2m) gene. PCR conditions were as follows: gitrl, 57℃ annealing temperature 33 cycles; β2m, 57℃ annealing temperature 27 cycles. Sequences of the PCR primers were as follows: gitrl forward, 5' CAA GTC CTC AAA GGG CAG AG 3'; gitrl backward, 5AGC TTC CCA TCA GAT GTC GT 3'; β2m forward, 5' CAG TGT GAG CCA GGA TAT AG 3'; β2m backward, 5' TGA CCG GCT TGT ATG CTA TC 3'.
RESULTS AND DISCUSSION
FLS induce Tregs to stop expressing Foxp3 in a contact-dependent manner
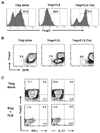
FLS colocalize with Tregs in the inflamed synovia of patients with RA. To examine whether the FLS affect the activities of the Tregs, we carried out FLS-Treg coculture experiments that mimicked in vivo inflamed synovia. To this end, Tregs that had been sorted to >95% purity were cultured in the presence or absence of FLS for 4 days and phenotypic changes were assessed by FACS. During growth without FLS, approximately 13% of the Tregs ceased to express Foxp3, and the fraction of ex-Foxp3 cells was greatly increased by coculture with FLS (Fig. 1A). Transwells physically separating the Tregs from the FLS interfered with this effect, suggesting that these two types of cell communicate via cell contact.
We examined other phenotypes of ex-Foxp3 cells cocultured with FLS. We found that these ex-Foxp3 cells expressed reduced levels of GITR (Fig. 1B). Unlike the ex-Foxp3 cells reported previously (10), the FLS-induced ex-Foxp3 cells did not produce IFN-γ or IL-17 (Fig. 1C). Thus, these results suggest that FLS instruct the Tregs to cease expressing Foxp3 and GITR in a contact-dependent manner. However this interaction seems to be insufficient to convert Tregs to effector T cells producing pathogenic cytokines. Since reduced Foxp3 expression attenuates the suppressive activity of Tregs (10), the FLS-mediated reduction in Foxp3 expression may be expected to impair the activity of Tregs in synovia.
The FLS-driven reduction of Foxp3 expression is inhibited by blocking antibody to GITR
GITR is a member of the TNF receptor family with homology to other members such as OX40 and 4-1BB (15). Expression of GITR is induced when naïve T cells are primed by antigenic challenge, and engagement of GITR with its ligand (GITRL) triggers costimulatory signals that enhance T cell proliferation and effector functions. In contrast, GITR is constitutively expressed at a high level on the surface of Tregs, and signals that it generates have been shown to reduce the suppressive activity and stability of Foxp3 expression (16,17). These findings led us to hypothesize that FLS signal Tregs through GITR to reduce Foxp3 expression.
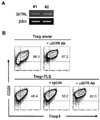
To test this idea we first examined by RT-PCR whether FLS express GITRL transcripts. Expression of GITRL transcripts was detected in both our lines of FLS derived from arthritic mice (Fig. 2A). Thus, this result suggests that FLS could be capable of signaling GITR+ Tregs.
To see if this was the case, we added anti-GITR mAb (DTA-1) to the cells. Originally this DTA-1 mAb was characterized as a GITR agonist that activated GITR+ effector T cells and suppressed Treg functions (16). However, in our system, the DTA mAb did not affect the proliferation or viability of Tregs cultured on their own, or their level of Foxp3 expression (data not shown, and Fig. 2B). However the DTA-1 mAb, but not an isotype-matched control mAb, prevented the FLS-induced reduction of Foxp3 expression by the Tregs. Therefore, it is likely that, rather than acting as a GITR agonist, the DTA mAb interferes with the interaction between GITRL and GITR, and that the GITR expressed on the surface of Tregs act as a sensor for a signal from the FLS.
The interaction between FLS and Tregs increases IL-6 production
Activated FLS produce a variety of proinflammatory cytokines, chemokines and matrix metalloproteases. They are the main source of IL-6 in inflamed joints, and levels of IL-6 are directly related to the severity of RA (18). IL-6 promotes inflammatory responses directly by activating diverse effector cells and indirectly by generating proinflammatory Th17 cells in the presence of TGF-β (19). It also acts in synergy with IL-1 to reduce the expression of Foxp3 (11,12). Therefore, IL-6-induction in synovia may be crucial for perpetuating inflammation and tissue injury.
We tested whether Treg-FLS coculture enhances IL-6 production. FLS cultured alone without Tregs spontaneously secreted a substantial amount of IL-6, but Tregs cultured alone did not. IL-6 increased markedly when the cells were cocultured, suggesting that contact with Tregs the production of IL-6 by the FLS (Fig. 3). Because interactions between cognate costimulators favor bidirectional signaling, it is possible that the engagement of GITR with GITRL signals the FLS to make IL-6. However it remains to be determined whether the IL-6 induction is actually mediated by the GITRL-GITR system rather than by an interaction between other surface molecules on the FLS and Tregs. In addition, we cannot rule out the possibility that it is the Tregs that produce the IL-6 in the context of crosstalk with the FLS, given that ex-Foxp3 cells have been shown to acquire the ability to produce other proinflammatory cytokines (10).
In summary, we have shown that the interaction between FLS and Tregs can cause harmful changes in both populations. As a result of their crosstalk, a substantial portion of Tregs convert to ex-Foxp3 cells and the FLS become more proinflammatory. The loss of Foxp3 expression appears to be mediated by the GITRL-GITR system. These results provide a novel mechanism by which the anti-inflammatory activity of Tregs is compromised by the proinflammatory milieu of rheumatoid synovia.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download