Abstract
Our previous report showed that polyacetylene compound, 1-Heptadecene-11, 13-diyne-8, 9, 10-triol (PA) from the root of Cirsium japonicum var. ussuriense has anti-inflammatory activity. In this study we investigated the role of the PA as inhibitor of caspase-1, which converts prointerleukin-1β (proIL-1β) to active IL-1β and is activated by inflammasome involved in the inflammatory process. We tested the effect of PA on the production of pro-inflammatory cytokines, IL-1β in murine macrophage cell line, RAW264.7. PA inhibited lipopolysaccharide (LPS)-induced IL-1β production by macrophages at a dose dependent manner. PA also suppressed the activation of caspase-1. The mRNA level of ASC (apoptosis-associated spec-like protein containing a CARD), an important adaptor protein of inflammasome, was decreased in the PA treated group. Therefore our results suggest that the anti-inflammatory effect of PA is due to inhibit the caspase-1 activation.
Pattern recognition receptors (PRRs) are essential components for the recognition of conserved microbial structure known as pathogen-associated molecular patterns (PAMPs) and stimulation of the production of pro-inflammatory cytokines in the innate immune system. PRRs can be classified into three types; toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) (1). Recently, NLRs play a key role in the surveillance of mammalian cytoplasm and in several biological processes, which include host defense against microbes and inflammation (2,3). The ultimate outcome of NLR signaling is to trigger a proinflammatory response by activation and secretion of cytokines via the NF-κB activation and the inflammasome (2,3).
IL-1β is a representative proinflammatory cytokine and is expressed after an inflammatory stimulus. TLRs activation by PAMPs results in the generation of pro-IL-1β via NF-κB activation. IL-1β requires a second signal resulting in caspase-1-mediated cleavage of pro-IL-1β to release the active molecule (4). The caspase-1 activation is regulated by a cytosolic protein complex called inflammasome, which consists of an NLR family member, the adaptor protein ASC (apoptosis-associated spec-like protein containing a CARD, caspase activation and recruitment domain), and caspase-1 (5).
During the research for novel anti-inflammatory agents from natural plant, we found that Heptadecene-11, 13-diyne-8, 9, 10-triol (PA) from the root of Cirsium japonicum var. ussuriense inhibits the production of proinflammatory cytokines, IL-1β and TNF-α, in macrophages stimulated by lipopolysaccharide (LPS) via suppression of NF-κB activation (6). In this study, therefore we assess whether the anti-inflammatory activity of PA in macrophages is associated with the expression of caspase-1 and ASC, which are involved in inflammasome.
1-Heptadecene-11, 13-diyne-8, 9, 10-triol (PA) was prepared from the root of Cirsium japonicum var. ussuriense as previously described (6). The purified PA was dissolved in dimethylsulfoxide (DMSO, Sigma co. St. Louis, USA) as stock solution and was used directly for cell culture treatment. The final concentration of DMSO was 1.0% in the culture media, which did not show any effect in the assay system.
RAW 264.7 cells, murine macrophage cell line, were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone) supplemented with 20 mM HEPES, 10% FBS, 100 U/ml of penicillin, and 100 µg/ml of streptomycin. The cells were pretreated with PA and/or caspase-1 inhibitor (Ac-YVAD-CMK, Calbiochem, Rockland, USA) for 1 h and then treated with LPS (100 ng/ml) from E. coli O111:B4 (Sigma co.) for indicated time.
Total RNA from LPS- or PA-treated RAW 264.7 cells was prepared using Trizol reagent and the amount of total RNA was quantified with spectrophotometer. cDNA was synthesized with SuperScript cDNA synthesis III kit (Invitrogen, Grand Island, USA) in accordance with the manufacturer's instructions. Quantitative RT-PCR was used to detect Caspase-1 and ASC transcripts using β2M as an endogenous control in RAW 264.7 cells. PCR amplification was performed with 2×QantiTect SYBR Green PCR Master mix (Qiagen, Valencia, USA) according to the manufacturer's protocol. The primers used in this study were as follows: Caspase-1; Sense, 5'-tgaaagaggtgaaagaatt-3', Anti-sense, 5'-tctccaagacacattatct-3', ASC; Sense, 5'-agacatgggcttacagga-3', Anti-sense, 5'-ctccctcatcttgtcttgg-3', β-actin; Sense, 5'-tggaatcctgtggcatggatgaaac-3', Anti-sense, 5'-taaaacgcagctcagtaacagtccg-3'. The PCR conditions were as follows: 95℃ for 15 minutes, followed by 40 cycles of 95℃ for 15 seconds, 55℃ (Caspase1, ASC, and β2M) for 30 seconds and 72℃ for 30 seconds. Levels of mRNA were measured by a Chromo 4 (MJ Research). For relative quantification, the expression of each gene was normalized to the expression of β2M in the cells relative to a calibrator. The amount of target was represented by 2-ΔΔCt.
Among groups, data and statistical analyses were carried out using SigmaStat, version 3.1, by one-way analysis of variance (ANOVA) or Kruskal-Wallis ANOVA, depending on normality of data. The significance was further confirmed by the Tukey test. Differences were considered significant when p was less than 0.05.
Inflammation is a complex but an essential protective response by the host immune system against physical, chemical, and infective agents. However, it is frequent that inflammatory response to several stimuli leads to the damaging of normal tissues (9-11). It is for this reason that inflammation is normally regulated by the body. Despite many efforts to develop anti-inflammatory drugs, there is still a large demand for developing new agents. To search for the novel therapeutic agents against inflammatory diseases, we examined anti-inflammatory activity of natural products from plants. Our previous study demonstrated that PA from Cirsium japonicum var. ussuriense has anti-inflammatory activities (6). PA especially inhibited the production of pro-inflammatory cytokines in macrophages activated with LPS. Therefore in this study we explained the exact mechanism of PA on its anti-inflammatory activity.
To confirm whether PA inhibits IL-1β, one of the pro-inflammatory mediators, we investigated the effect of PA on LPS-induced IL-1β production in RAW 264.7 cells. The result showed that PA significantly inhibited LPS-induced IL-1β production by macrophages at a dose dependent manner (Fig. 1A), consistent with the previous result (6).
Pro IL-1β requires cleavage to become biologically active IL-1β via caspase-1 (4). Therefore we determined whether the inhibitory effect of PA on IL-1β production in RAW cells activated with LPS was due to suppression of caspase-1 activity by PA. As shown in Fig. 1B, LPS-induced caspase-1 activation was decreased by treatment of PA at a dose dependent manner, consistent with the result on IL-1β production (Fig. 1B). Suppression of PA on caspase-1 activation also compared to that of Ac-YVAD-CMK, a caspase-1 inhibitor. In the presence of Ac-YVAD-CMK, there was a marked decrease in IL-1β production (Fig. 1C) and caspase-1 activity (Fig. 1D). Consistent with these results, pre-treatment of PA suppressed IL-1β secretion and caspase-1 activation in macrophages (Fig. 1C and D).
Recently, the molecular components responsible for caspase-1 activation have been identified (12). These consist of a family of cytosolic protein complex called the inflammasome consisting of a NALP, ASC, and caspase-1. Therefore we examined the effect of PA on the expression of ASC and caspase-1 mRNA. To test whether PA has a role in the regulation of caspase-1, we quantified caspase-1 transcripts following LPS treatment in the presence or absence of PA in the macrophages. There was a significant increase in caspase-1 transcript on early time, 2 hours after LPS treatment. However, treatment of PA reduced the expression of caspase-1 (Fig. 2A). To establish that the reduction of caspase-1 expression was associated with decreased the ASC expression we next tested the effect of PA on the regulation of ASC mRNA expression. The result showed that treatment of PA significantly reduced the expression of ASC, while LPS up-regulated ASC transcript immediately at 2 hours after treatment (Fig. 2B).
IL-1β is a multifunctional cytokine that is responsible for mediating a variety of processes in the host defense response, inflammation, and so on. Macrophages and many other cell types produce IL-1β by the action of stimuli such as LPS, the cell wall component from gram negative bacteria. Since IL-1β is a highly potent pro-inflammatory cytokines, agents that suppress its production and/or activity might be of a particular pharmacological and clinical interest. As cytokines are critical to the pathogenesis of inflammatory disorders, inhibition of their production provides therapeutic targets in various inflammatory diseases. In the present study, we demonstrated that PA significantly inhibits LPS-induced transcript of genes involved in inflammasome, which is complex protein and is responsible for activation of inflammatory processes (12).
Although we showed that PA suppressed the increase in mRNA level of caspase-1 and ASC triggered by LPS, it is still possible that PA could directly inhibit the inflammasome activation beyond transcriptional control. Therefore the other possible mechanism of PA for inflammasome activation needs to be elucidated in our future study.
Again, caspase-1 is a cysteine protease originally cloned as IL-1β-converting enzyme (5,13). The release of IL-1β protein is dependent on the activation of caspase-1. Since caspase-1 is an essential regulator of inflammatory responses through its capacity to process and activate proIL-1β, its inhibition by PA may offer a possible approach to the prevention or treatment of severe inflammatory diseases. Furthermore caspase-1 may be of interest to us as a candidate molecule for treatment of inflammatory disease.
Figures and Tables
Figure 1
IL-1β production and caspase-1 activation by LPS were inhibited with treatment of PA in RAW 264.7 cells. (A, B) RAW264.7 cells were plated on 12 well cell culture plate, treated with PA (A, B) at a dose dependent manner (12.5~100 µg/ml). (C, D) In some experiments, the cells were treated with PA (50 µg/ml) or caspase-1 inhibitor, Ac-YVAD-CMK (C, D, 20 uM) for 1 hr, and then with LPS (100 ng/ml). After 24 h of these treatments, cell culture supernatants and cell lysates were collected and assayed for IL-1b secretion and caspase-1 activity, respectively. The data are representative of at least three independent experiments, each done in triplicate; *p<0.05, **p<0.01 compared to control groups treated with LPS alone.

Figure 2
The expression of genes (A: caspase-1; B: ASC) involved in the inflammasome in RAW 264.7 cells treated with PA (50 µg/ml) and LPS (100 ng/ml) by Real-time PCR. The data are representative of at least three independent experiments, each done in triplicate; *p<0.05 compared to control groups treated with LPS alone.
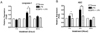
References
1. Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004. 41:1099–1108.

2. Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Núñez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008. 10:1–8.

3. Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007. 27:549–559.

4. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995. 267:2000–2003.

5. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007. 7:31–40.

6. Kang TJ, Moon JS, Yim DS, Lee SK. Polyacetylene compound from Cirsium japonicum var. ussuriense inhibits the LPS-induced inflammatory reaction via suppression of NF-κB activity in RAW 264.7 cells. Biomol Ther. 2011. 19:97–101.

7. Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008. 38:1574–1584.

8. Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002. 70:6896–6903.

11. Cho W, Park SJ, Shin JS, Noh YS, Cho EJ, Nam JH, Lee KT. Anti-inflammatory effects of the methanol extract of Polytrichum Commune via NF-κB inactivation in RAW 264.7 cells. Biomol Ther. 2008. 16:385–393.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download