Abstract
It has been reported that vitamin C plays an effective role in the treatment and prevention of cancer, but its specific mechanisms are still largely unknown. The incidence of colon cancer is now increasing in Korea. Therefore, we have examined here the effect of vitamin C on the induction of the apoptosis on colon cancer and its related mechanisms. We have found that remarkable increase of the apoptosis and the calcium influx in endoplasmic reticulum (ER) in human colon cancer cell line, HCT-8. However, vitamin C-induced apoptosis was effectively inhibited by the pre-treatment of BAPTA-AM (1,2-bis(o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid), which is well-known as a calcium specific chelator. During the apoptosis, we found the increase of the translocation of Bad to mitochondria from cytosol, after releasing from 14-3-3β. In this process, the expression of Bax, a well-known pro-apoptotic protein, was also increased. Taken together, vitamin C induces apoptosis of colon cancer cell line, HCT-8 through the increase of 1) the calcium influx in endoplasmic reticulum (ER), 2) the translocation of Bad to mitochondria, and 3) the expression of Bax.
Although vitamin C is a well-known anti-oxidant as well as an essential nutrient, there are numerous reports regarding its tumoricidal effects. It induces apoptosis via the disruption of mitochondrial membrane potential and the suppression of the translocation of transferring receptor from cytosol to membrane (1,2). In addition, vitamin C suppresses the proliferation of cancer cells through the growth arrest at G1 stage that is closely related with the modulation of the activity of p53-p21Waf1/Cip1 and CDK2 (3-5). The productions of factors that are involved in the metastasis are also down-regulated by vitamin C treatment (6,7). In our previous report, vitamin C increased the immune susceptibility of stomach cancer via the increase of Fas and MHC I (8).
Colon cancer has now become one of the most common cancers in the western countries as well as North East Asia including Korea (8). Chemotherapeutic reagents, such as 5-fluorouracil oxaliplatin, leucovorin and irinotecan, were used to prevent the recurrence of colon cancer (9-11). It is reported that some nonsteroidal anti-inflammatory drugs (NSAIDs) including sulindac and aspirin are quitely efficacious as a chemo-preventing drugs, but the side effects are still not clearly identified (12,13). In this point of view, vitamin C is one of the best substances, which acts not only as a chemopreventing agent but also therapeutic agent against colon cancer.
It is known that there are two different apoptosis; one is death receptor dependent (the extrinsic) and the other is death receptor-independent mitochondrial pathway (the intrinsic) (14,15). The former is usually initiated by TNF receptor or its superfamily, such as CD95 (16,17). In contrast, apoptosis via receptor-independent mitochondrial death pathway is usually triggered by chemicals and p53 (18). In addition, Bcl-2 family members, such as Bid, Bax, and Bad, are key mediators in this pathway (19). In several recent reports, endoplasmic reticulum (ER) plays an important role in receptor-independent death pathway (20-22). Since ER is sensitive to disturbance of cellular energy level, the redox state or intracellular Ca2+ level, such stresses are closely related with the cell death caused by the chaining on ER homeostasis (23,24). The releasing of calcium from ER to cytosol induces apoptosis that is followed by the translocation of Bad to mitochondria in a dependent of the activation of calcineurin, Ca2+/calmodulin serine/threonine phosphatase (25-27). Bad is phosphorylated by survival factors and sequestered in the cytosol as a complex with signal transducer protein, 14-3-3. However, it is translocated to mitochondria and bound to Bcl-2 and Bcl-xL, after being dephosphorylated and dissociated from 14-3-3 by apoptotic signals (28,29).
We presented here the novel apoptosis mechanism of vitamin C through the increasing of ER stress and the translocation of Bad to mitochondria after dissociation from 14-3-3β in human colon cancer cell line, HCT-8.
Human colon cancer cell line, HCT-8, was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in continuous log phase growth and cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS).
After cells (2×106) were exposed to various concentrations of vitamin C (0.25, 0.5, 1, 2 and 4 mM) for 24 hrs, they were collected and washed twice with cold PBS, and then resuspended in 1× binding buffer at a concentration of 1×106 cells/ml. Cells were then incubated with 5 µl of FITC conjugated Annexin V (BD Pharmingen, San Diego, CA, USA) at room temperature for 15 min in the dark. One microliter of 7-AAD (BD Pharmingen, San Diego, CA, USA) was added prior to flow cytometric analysis by FACSCaliber (BD Pharmingen, San Diego, CA, USA).
Cells (2×106) were incubated in the presence or absence of 2 mM vitamin C for 0.5, 1, and 2 hrs in RPMI containing 1% fetal bovine serum. The single cell suspension (1×106) was loaded with 0.5 µM Fluo-3/AM (Molecular Probes, Carlsbad, CA, USA) in 200 µl of RPMI medium without serum for 30 min at 37℃. Cells were then washed twice serum-free medium and resuspended. Samples were analyzed for Ca2+-dependent increase in Fluo-3 fluorescence emission by a flow cytometric analysis (excitation: 485 nm, emission: 538 nm).
Cells (2×106) were exposed to 2 mM of vitamin C for 3, 6 and 9 hrs and then mitochondrial fractions were prepared by a Mitochondria Isolation Kit according to the manufacturer's instructions (Pierce, Rockford, IL, USA). The mitochondrial fractions were purified by two-step gradient centrifugation and stored at -4℃ for further analysis by immunoblotting to investigate the translocation of Bad from cytosol to mitochondria.
The cells were lysed and proteins extracted in a buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor cocktail. The protein concentration was measured using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein (30~80 µg) were resolved on 12% polyacrylamide-SDS gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk and 0.1% Tween 20-phosphate buffered saline (PBS) for 1 hr, washed with 0.1% Tween 20-PBS, and then exposed to primary antibody for overnight at 4℃. Anti-Bax, and 14-3-3σ (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-14-3-3β and anti-Bad (Upstate Biotechnology, Lake Placid, NY, USA); anti-Cytochrome c (BD Biosciences, San Jose, CA, USA) were diluted 1:200~1,000 in 0.1% Tween 20-PBS. After washing, blots were exposed to biotin-conjugated secondary antibodies (1:5,000) for 1 hr at RT. The membranes were then washed, incubated with a 1:5,000 diluted streptavidin-horseradish peroxidase and immunoreactive proteins were visualized with the ECL detection system (Amersham Biosciences Corp., Piscataway, NJ, USA).
Based on our previous reports that relatively high concentration of vitamin C induced apoptosis on tumor cells (1,2), we investigated whether vitamin C could also induce apoptosis in human colon cancer cell line, HCT-8. As shown in Fig. 1A, we found the extensive apoptosis was induced by the treatment of 2 mM of vitamin C for 24 hrs, and apoptosis was shown from 12 hrs after treatment of vitamin C (Fig. 1B).
There are several reports that the increase of ER membrane permeability and calcium releasing from ER are related with the induction of apoptosis in tumor (20-24). Therefore, the cytosolic calcium level was examined to clarify whether the induction of apoptosis in HCT-8 by the treatment of vitamin C is also related with the changing of alteration on ER. As we expected, cytosolic calcium levels in vitamin C-treated HCT-8 were increased from 30 min and it was peaked at 2 hrs after vitamin C treatment (Fig. 2A). To examine that the increase of calcium in cytosol from ER is related with the apoptosis of HCT-8 by vitamin C, the cells were pre-treated with BAPTA-AM (1,2-bis (o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid) for 30 min prior to treatment of vitamin C. We found that the apoptosis in HCT-8 by 2 mM of vitamin C was effectively suppressed (Fig. 2B).
Since we have already shown that vitamin C effectively induced the apoptosis on HCT-8 and it is related with the stress on ER, we investigated the change on the expression of pro-apoptotic Bcl-2 family member, Bax. In addition, the dissociation of Bad from 14-3-3β that is affected by ER stress was investigated. Bax expression was remarkably increased at 6 hrs after vitamin C treatment (Fig. 3A). At the same time, the expression of Bad was also increased, but there were no remarkable changing on the expression of 14-3-3β and 14-3-3σ two of major subunit of 14-3-3 proteins (Fig. 3B). The increase of cytosolic calcium released from ER is responsible for the dissociation ofBad from 14-3-3 (25-27). Therefore, we examined the amounts of Bad that is bound with 14-3-3β in the cytosol after treatment of vitamin C, after immunoprecipitation by using of anti 14-3-3β antibody. Interestingly, the amount of associated Bad with 14-3-3β in the cytosol was decreased at 6 hrs after vitamin C treatment (Fig. 3C).
To investigate whether the dissociated Bad from 14-3-3β is translocated to mitochondria, the localization of Bad in mitochondria was examined by immunoblotting after separation of mitochondrial protein fraction from HCT-8 in the presence of vitamin C. As we expected, the relative amount of Bad was increased in mitochondrial fraction, in a time dependent manner of vitamin C treatment (Fig. 4A). Immunoblotting against cytochrome C was performed to distinguish mitochondrial fraction from cytosolic fraction. The amount of Bad in mitochondria upon vitamin C treatment was presented as the relative ratio of Bad and cytochrome C (Fig. 4B).
Most mammals can synthesize vitamin C by conversion of glucose into ascorbate in liver by L-gulono-γ-lactone-oxidase (Gulo). However, human and some primates cannot synthesize vitamin C due to the mutation of gene encoding L-gulono-γ-lactone-oxidase (30,31). It has shown that vitamin C has various biomedical efficacies such as anti-inflammation, immune modulation and antioxidant in the immune system and the central nerve system (32,33). In cancer therapy, it is thought that vitamin C has a great potential on the reduction of the side effects of chemotherapeutic drugs as well as the increasing of therapeutic efficacy. It has been reported that vitamin C prevents proliferation and metastasis of cancer cells (4,6,34). Moreover, it could induce apoptosis in cancer cells via the disruption of mitochondrial membrane potential (1). It is well-known mitochondria and ER are important intracellular organelles during the TNF-receptor independent apoptosis pathway, but the specific action mechanisms of vitamin C on the induction of apoptosis via the modulation of ER function.
Bax plays an important role in induction of apoptosis through the releasing of cytochrome C from mitochondria (19). Besides Bax, several types of Bcl-2 family proteins, such as Bid and Bad, are involved in this process. Bid is a mediator in a TNF-receptor dependent apoptosis pathway, but Bad acts in TNF-receptor independent apoptosis pathway after dissociation from 14-3-3 protein (25-29). As shown in Fig. 3A, Bax expression was increased by the treatment of vitamin C, even without the stimulation of TNF-receptors on HCT-8. Therefore, the mechanisms on increasing Bax expression by vitamin C should be further investigated. We have also found that the remarkable dissociation of Bad from 14-3-3β is by vitamin C (Fig. 3B). As we previously described, the disruption of mitochondrial potential without caspase-8 activation is major pathway on the induction of apoptosis in B16F10 (1). Therefore, it seems that dissociated Bad from 14-3-3β is key mediator in the vitamin C-induced apoptosis through the disruption of mitochondrial membrane potential.
It is known that the stress to endoplasmic reticulum (ER) by an alteration of calcium concentration or redox state induces apoptosis (20-24). The releasing of Ca2+ from ER is an important event for dissociation of Bad from 14-3-3β (28,29). As we showed in Fig. 2A, cytosolic Ca2+ concentration is increased by vitamin C treatment. We observed that BAPTA-AM, specific Ca2+ chelator, was effectively inhibited vitamin C-induced apoptosis (Fig. 2B). Therefore, the releasing of Ca2+ from ER to cytosolic in HCT-8 is the initial step in the apoptosis by the treatment of vitamin C. Taken together, vitamin C effectively induces the apoptosis in human colon cancer cell line, HCT-8 through the calcium influx in ER. And it is followed by the translocation of Bad to mitochondria after dissociation from 14-3-3β. Finally, it seems that translocated Bad is involved in the apoptosis after heterodimerization with Bcl-2 or Bcl-xL proteins to promote mitochondrial dependent apoptosis (18,22).
Human colon cancer cell line HCT-8 is resistant to chemotherapeutic drugs like 5-fluorouracil and cisplatin (35). However, we presented that vitamin C has efficacy on the induction of apoptosis in HCT-8 (Fig. 1). It suggests that vitamin C is a useful adjuvant for the treatment of colon cancer with chemotherapeutic drugs. The synergistic or additive effect of combination therapy of chemotherapeutic drugs and vitamin C is now under investigation.
Figures and Tables
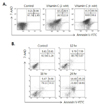 | Figure 1Dose and time kinetic study of vitamin C for induction of apoptosis in human colon cancer cell line, HCT-8. (A) Cells (2×106) were incubated in the presence of 2 and 4 mM of vitamin C for 24 hrs. (B) Cells (2×106) cells were incubated in the presence of 2 mM of vitamin C and incubated for 12, 18 and 24 hrs. Then the cells were collected and the effect of vitamin C on induction of apoptosis was measured by Annexin V-FITC/7-AAD staining. The result is representative of three experiments. |
 | Figure 2Apoptosis of HCT-8 via the increase of intracellular calcium level by the treatment of vitamin C. (A) Cells (1×106) were incubated in the abscence or presence of 2 mM of vitamin C for 0.5, 1 and 2 hrs, and then loaded with 0.5 µM of Fluo-3/AM at 37℃. Each sample was analyzed for detection of increased cytosolic calcium levels through changing in fluorescence of the dyes using the standard filters following a 488 nm excitation. (B) Cells were pre-incubated for 30 min with (B) BAPTA-AM (1.25 µM), respectably, prior to treatment with 2 mM of vitamin C for 24 hrs. And then cells were collected and the effect of vitamin C on induction of apoptosis was measured by Annexin V-FITC/7-AAD staining. Result is representative of three experiments. Data present as Mean±SD. |
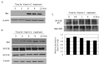 | Figure 3Increase of Bax expression and the dissociation of Bad expression from 14-3-3β by the treatment of vitamin C. (A and B) Cells (2×106) were incubated for 3, 6, 9 and 12 hrs in the presence or absence of 2 mM of vitamin C. Then protein was extracted and subjected to immunoblotting done by using (A) anti-Bax antibodies and (B) anti-Bad, anti-14-3-3β and anti-14-3-3σ antibodies as described in materials and methods. The result is representative of more than three experiments. (C) After cells were cultured in the presence or absence of 2 mM of vitamin C for 0.5, 1, 3, and 6 hrs, protein was extracted and subjected to immunoprecipitation with a monoclonal antibody against 14-3-3β. The immunoprecipitatedprotein was used to analyze for detection of associated Bad by immunobloting. The blotwas stripped out and reprobed by anti 14-3-3β antibody as described in materials and methods. The result is representative of more than three experiments. The density of each band was measured by densitometry, and the values were expressed as the ratio Bad/14-3-3β. |
 | Figure 4The translocation of Bad from 14-3-3β to mitochondria by the treatment of vitamin C. (A) Cells (2×106) was incubated for 3, 6 and 9 hrs in the presence or absence of 2 mM of vitamin C. Mitochondrial fraction was prepared as described in materials and methods. Then western blotting was done by using antibodies against Bad and cytochrome C. (B) The density of each band was measured by densitometry, and the values were expressed as the ratio Bad/Cytochrome C. |
ACKNOWLEDGEMENTS
This work was supported by the grants from the National R&D Program for Cancer Control (Grant # 1020030), Ministry of Health, Welfare & Family. Affairs to Jae Seung Kang.
References
1. Kang JS, Cho D, Kim YI, Hahm E, Yang Y, Kim D, Hur D, Park H, Bang S, Hwang YI, Lee WJ. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003. 52:693–698.

2. Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005. 204:192–197.

3. Kim JE, Jin DH, Lee SD, Hong SW, Shin JS, Lee SK, Jung DJ, Kang JS, Lee WJ. Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int J Mol Med. 2008. 22:651–655.

4. Hahm E, Jin DH, Kang JS, Kim YI, Hong SW, Lee SK, Kim HN, Jung da J, Kim JE, Shin DH, Hwang YI, Kim YS, Hur DY, Yang Y, Cho D, Lee MS, Lee WJ. The molecular mechanisms of vitamin C on cell cycle regulation in B16F10 murine melanoma. J Cell Biochem. 2007. 102:1002–1010.

5. Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008. 216:180–188.

6. Kim HN, Kim H, Kong JM, Bae S, Kim YS, Lee N, Cho BJ, Lee SK, Kim HR, Hwang YI, Kang JS, Lee WJ. Vitamin C down-regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J Cell Biochem. 2011. 112:894–901.

7. Cho D, Hahm E, Kang JS, Kim YI, Yang Y, Park JH, Kim D, Kim S, Kim YS, Hur D, Park H, Pang S, Hwang YI, Lee WJ. Vitamin C downregulates interleukin-18 production by increasing reactive oxygen intermediate and mitogen-activated protein kinase signalling in B16F10 murine melanoma cells. Melanoma Res. 2003. 13:549–554.

8. Zhang J, Dhakal IB, Zhao Z, Li L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur J Cancer Prev. 2012. 21:480–489.

9. Ruiz de Erenchun F. The role of new agents in the treatment of colorectal cancer. Oncology. 2005. 68:162.

10. Inoue Y, Miki C, Watanabe H, Hiro J, Toiyama Y, Ojima E, Yanagi H, Kusunoki M. Schedule-dependent cytotoxicity of 5-fluorouracil and irinotecan in a colon cancer cell line. J Gastroenterol. 2006. 41:1149–1157.

11. Pallis AG, Mouzas IA. Adjuvant chemotherapy for colon cancer. Anticancer Res. 2006. 26:4809–4815.
12. Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, Lubet RA, Kelloff GJ, Verma A, Moser AR, Dove WF. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996. 56:710–714.
13. Liou JY, Ghelani D, Yeh S, Wu KK. Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell apoptosis by suppressing 14-3-3epsilon. Cancer Res. 2007. 67:3185–3191.

14. McDonnell MA, Wang D, Khan SM, Vander Heiden MG, Kelekar A. Caspase-9 is activated in a cytochrome c-independent manner early during TNFalpha-induced apoptosis in murine cells. Cell Death Differ. 2003. 10:1005–1015.

15. Katoh I, Tomimori Y, Ikawa Y, Kurata S. Dimerization and processing of procaspase-9 by redox stress in mitochondria. J Biol Chem. 2004. 279:15515–15523.

16. Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995. 3:673–682.

17. Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003. 22:8628–8633.

18. von Haefen C, Wieder T, Gillissen B, Stärck L, Graupner V, Dörken B, Daniel PT. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002. 21:4009–4019.

19. Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005. 4:1–11.
20. Boya P, Cohen I, Zamzami N, Vieira HL, Kroemer G. Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. Cell Death Differ. 2002. 9:465–467.

21. Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006. 281:7260–7270.
22. Zhang D, Armstrong JS. Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ. 2007. 14:703–715.

23. Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, Lai MD. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004. 279:46384–46392.

24. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006. 7:880–885.

25. Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003. 22:8608–8618.

26. Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis. Biochem J. 2004. 379:805–813.

27. Cardoso SM, Oliveira CR. The role of calcineurin in amyloid-beta-peptides-mediated cell death. Brain Res. 2005. 1050:1–7.
28. Rapp UR, Fischer A, Rennefahrt UE, Hekman M, Albert S. BAD association with membranes is regulated by Raf kinases and association with 14-3-3 proteins. Adv Enzyme Regul. 2007. 47:281–285.

29. Claerhout S, Decraene D, Van Laethem A, Van Kelst S, Agostinis P, Garmyn M. AKT delays the early-activated apoptotic pathway in UVB-irradiated keratinocytes via BAD translocation. J Invest Dermatol. 2007. 127:429–438.

30. Levine M, Dhariwal KR, Welch RW, Wang Y, Park JB. Determination of optimal vitamin C requirements in humans. Am J Clin Nutr. 1995. 62:6 Suppl. 1347S–1356S.

31. Wolucka BA, Communi D. Mycobacterium tuberculosis possesses a functional enzyme for the synthesis of vitamin C, L-gulono-1,4-lactone dehydrogenase. FEBS J. 2006. 273:4435–4445.

32. Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004. 27:101–106.

33. Hartel C, Puzik A, Gopel W, Temming P, Bucsky P, Schultz C. Immunomodulatory effect of vitamin C on intracytoplasmic cytokine production in neonatal cord blood cells. Neonatology. 2007. 91:54–60.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download