Abstract
Dioscoreae Rhizome (DR) has been used in traditional medicine to treat numerous diseases and is reported to have anti-diabetes and anti-tumor activities. To identify a bioactive traditional medicine with anti-inflammatory activity of a water extract of DR (EDR), we determined the mRNA and protein levels of proinflammatory cytokines in macrophages through RT-PCR and western blot analysis and performed a FACS analysis for measuring surface molecules. EDR dose-dependently decreased the production of NO and pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and PGE2, as well as mRNA levels of iNOS, COX-2, and pro-inflammatory cytokines, as determined by western blot and RT-PCR analysis, respectively. The expression of co-stimulatory molecules such as B7-1 and B7-2 was also reduced by EDR. Furthermore, activation of the nuclear transcription factor, NF-κB, but not that of IL-4 and IL-10, in macrophages was inhibited by EDR. These results show that EDR decreased pro-inflammatory cytokines via inhibition of NF-κB-dependent inflammatory protein level, suggesting that EDR could be a useful immunomodulatory agent for treating immunological diseases.
Inflammation is part of the biological response to infection, irritation, or injury and is characterized by an influx of white blood cells, redness, heat, swelling, pain, and dysfunction of the organs involved (1,2). Macrophages and lymphocytes are activated in inflammation; these cells can produce pro-inflammatory mediators, nitric oxide (NO), pro-inflammatory cytokines, prostaglandin E2 (PGE2) or chemokines, and free radicals that enhance inflammation (3).
Macrophages play a part in both innate immunity and adaptive immunity, and their role is to stimulate lymphocytes to respond to pathogens (4). In response to endotoxins such as lipopolysaccharide (LPS), macrophages release pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α through the activation of nuclear factor (NF)-κB. Inducible nitric oxide (NO) synthase (iNOS) (5,6) and cyclooxygenase-2 (COX-2) (7) are also required the expression of NF-κB.
The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α modulate the immune system, but overexpression of inflammatory mediators induces the pathogenesis of several diseases such as atherosclerosis, rheumatoid arthritis, chronic inflammation, and autoimmune diseases (8-10). The anti-inflammatory cytokines IL-4 and IL-10 regulate the pro-inflammatory activity that results in immune-mediated diseases.
Nuclear factor-κB (NF-κB), transcription factor, controls the expression of pro-inflammatory mediators such as iNOS, COX-2, TNF-α, IL-1β, and IL-6 (11). It is chronically active in many inflammatory diseases, and NF-κB is therefore currently a target for treating various diseases (12). NF-κB is involved in cellular responses to inflammatory stimuli such as stress, bacterial or viral antigen, and cytokines (13). In its normal state, NF-κB consists of p50, p65, and IκBα subunits in the cytosol as an inactive form. The activity of NF-κB is primarily regulated by interaction with inhibitory IκBα proteins. After degradation of IκBα, free NF-κB (p50-p65) enters the nucleus and activates gene expression.
Dioscoreae Rhizome (DR) is a member of the Dioscoreaceae or Yam family and has been frequently used to cure diarrhea, cough, spermatorrhea, leukorrhea and frequency of urination and arthritis (14). Several studies have shown that DR decreases damage in renal tubules, inflammation in the central vein, and necrosis in the liver through its anti-inflammatory action (15). In addition, the inhibitory effect of Dioscorealide B (DB), a naphthofuranoxepin isolated from DR, on NO and TNF-α production was reported (16). However, the molecular anti-inflammatory mechanisms in macrophages remain unclear. Based on other data, we hypothesized that water extract of DR (EDR) may exert significant anti-inflammatory activity through the inhibition of inflammatory mediator production at the transcriptional level. In the present study, the direct effects of EDR on the production of inflammatory mediator expression and activation of the transcription factor have been examined.
Lipopolysaccharide (LPS) and XTT (sodium 3-[1-(phenylaminocarbonyl)-3, 4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate) were purchased from Sigma (St. Louis, MO, USA). Dulbecco's Modified Eagle's Medium (DMEM), antibiotic-penicillin/streptomycin solution, and fetal bovine serum (FBS, Hyclone, Logan, UT, USA) were used for the cell culture.
Dioscoreae Rhizoma was identified by Seungjeong Lee (College of Pharmacy, Chungbuk National University). Fifty grams of Dioscoreae Rhizoma were extracted with distilled deionized water (DDW) at 100℃. The water extracts were concentrated with a vacuum evaporator and then lyophilized.
RAW264.7 mouse macrophage cells (American Type Culture Collection, Manassas, VA, USA) were maintained in DMEM supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin, and 100 g/ml of streptomycin at 37℃ in a 5% CO2 incubator.
A commercially-available cell viability assay was employed to evaluate the cytotoxic effect of EDR using XTT (sodium 3-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate). RAW264. 7cells (2×105 cells/well) were plated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in 96-well micro-titer plates (Nunc, Roskilde, Denmark) and then cultured overnight at 37℃ in a 5% CO2 incubator. Afterwards, 50µl of XTT solution was added to each well for 10 hrs at 37℃ in a 5% CO2 incubator and the optical density (OD) was measured at 490 nm by a microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA).
The amount of nitrite produced by mouse macrophages was measured in cell culture supernatant. The cells were plated at a density of 1×106 cells in 200µl of culture medium per well in a flat-bottomed, 96-well plate. They were then treated with various concentrations of EDR in the absence of LPS (100 ng/ml) and incubated overnight. NO production was determined according to the method reported by Stuehr and Nathan (17). The amount of nitrite was measured using Griess reagent [stock-I: 0.2% N-(1-naphthyl) ethylenediamine-HCl, stock-II: 2% sulfanilamide in 5% H2PO4].
The amounts of IL-1β, IL-6, TNF-α, and PGE2 in the cell culture supernatant were measured using an ELISA kit (eBioscience, San Diego, CA, USA, R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. RAW 264.7 cells were cultured in DMEM with 10% FBS in 12-well, flat-bottomed plates at a density of 5×105 cells/well. The cells were treated with various concentrations of EDR in the absence or presence of LPS (100 ng/ml) at 37℃ for 48 hrs in humidified air with 5% CO2. Subsequently, the culture supernatant was collected and assayed according to the manufacturer's instructions.
Total RNA was extracted from RAW264.7 cells using the RNeasy Mini kit (QIAGEN, Valencia, CA, USA) in an RNase-free environment. RNA was quantified by reading the absorbance at 260 nm as previously described (14). The reverse transcription of 1µg RNA was carried out using M-MLV reverse transcriptase (Promega, Madison, WI, USA), oligo (dT) 16 primer, dNTP (0.5µM), and 1 U RNase inhibitor. After incubation at 65℃ for 5 min and 37℃ for 60 min, M-MLV reverse transcriptase was inactivated by heating at 70℃ for 15 min. The polymerase chain reaction (PCR) was performed in 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, and 2.5 mM dNTPs with 5 units of Taq DNA polymerase and 10 pM of each primer set for iNOS, COX-2, IL-1β, IL-6, and TNF-α. The cDNA was amplified by 35 cycles of denaturing at 94℃ for 45 s, annealing at 62℃ for 45 s, and extension at 72℃ for 1 min. The final extension was performed at 72℃ for 5 min. The PCR products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide. The primer sequences were as follows: 5' AGCTCCTCCCAGGACCACAC 3' (forward) and 5' ACGCTGAGTACCTCATTGGC 3' (reverse) for iNOS, 5' AAGAAGAAAGTTCATTCCTGATCCC 3' (forward) and 5' TGACTGTGGGAGGATACATCTCTC 3' (reverse) for COX-2, and 5' CAGGATGAGACATGACACC 3' (forward) and 5' CTCTGCAGACTCAAACTCCAC 3' (reverse) for IL-1β, 5' GTACTCCAGAAGACCAGAGG 3' (forward) and 5' TGCTGGTGACAACCACGGCC 3' (reverse) for IL-6, 5' TTGACCTCAGCGCTGAGTTG 3' (forward) and 5' CCTGTAGCCCACGTCGTAGC 3' (reverse) for TNF-α, 5' GTGGGCCGCCCTAGGACCAG 3' (forward) and 5' GGAGGAAGAGGATGCGGCAGT 3' (reverse) for β-actin. β-Actin was used as an internal control.
Cultured cells were collected, washed twice with cold PBS, and then suspended in hypotonic buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.2 mM PMSF, 0.5 mM DTT, 10µg/ml aportinin). After 15 min incubation on ice, the cells were lysed by the addition of 0.1% NP-40 and vigorous vortexing for 1 min. The nuclei were pelleted by centrifugation at 12,000×g for 1 min at 4℃ and resuspended in high salt buffer (20 mM HEPES, pH 7.9, 25% glycerol, 400 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate). The supernatant fluid was stored in aliquots at -70℃.
RAW 264.7 cells were washed with phosphate-buffered saline (PBS) and lysed with lysis buffer (1% SDS, 1.0 mM sodium vanadate, 10 mM Tris-Cl buffer, pH 7.4) for 5 min. 20µg of protein from the cell lysates was applied to 8~12% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk in PBST solution for 1 hr. They were then incubated with anti-IL-1β, anti-IL-6, anti-TNF-α, anti-IL-4, anti-IL-10, anti-iNOS, anti-COX-2, anti-p-IκBα or anti-NF-κB monoclonal antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2 hrs and washed 3 times with PBST. After incubation with an alkaline phosphatase-labeled secondary antibody (Abcam, Cambridge, MA, USA) for 2 hrs, the bands were visualized using a Western Blot Kit with alkaline phosphatase substrate (Vector, Burlingame, VT, USA).
RAW 264.7 cells (1×106 cells/ml) were cultured in Petri dishes. The cells were treated with various concentrations of EDR in the absence or presence of LPS (100 ng/ml). The dishes were incubated at 37℃ for 24 hrs in humidified 5% CO2 incubator under standard conditions. The cells were then washed with PBS. The washed cells were blocked with staining buffer containing 10% normal mouse serum (NMS) for 20 min on ice. The blocked cells were incubated with co-stimulatory molecules such as B7-1 and B7-2 antibody (BD Biosciences, San Jose, CA, USA) for 20 min on ice. The incubated cells were washed three times with staining buffer and then fixed by 1% paraformaldehyde in PBS. The fixed cells were measured by flow cytometry (Beckman Coulter, Brea, CA, USA).
To rule out any toxic effects of EDR, we tested its effect on the viability of RAW 264.7 cells by XTT assay. The exposure of cells to EDR (25~200 µg/ml) revealed no significant toxic adverse effects on viability compared to the untreated control.
To analyze the potential pro-inflammatory properties of EDR, we used murine macrophages, which produce NO. LPS was used as a positive control for macrophage activation. In LPS (100 ng/ml)-stimulated RAW 264.7 cells, iNOS increased NO production. Various concentrations of EDR (25, 50, 100, 200 µg/ml) were added to the culture media at the time of cell stimulation, results indicated that macrophages did not release NO in response to the medium alone; LPS-induced NO production decreased in an EDR concentration-dependent manner (Fig. 1A). Furthermore, EDR also dose-dependently decreased PGE2 production (Fig. 2A). EDR had a direct effect on pro-inflammatory mediators (NO and PGE2) related to modulation of the expression of iNOS and COX-2 according to RT-PCR and western blot analyses. As shown in Fig. 1B and Fig. 2B, EDR decreased the gene expression of LPS-induced iNOS and COX-2 in a dose-dependent manner. Furthermore, western blot analysis revealed that the expression of the iNOS and COX-2 levels was correlated with their gene levels (Fig. 1C and 2C).
To determine whether EDR had a direct effect on cytokine production, IL-1β, IL-6, and TNF-α secretion were measured in the macrophage cell line using the cytokine ELISA kit. IL-1β, IL-6, and TNF-α are the major pro-inflammatory cytokines produced by monocytes and macrophages. As shown in Fig. 3A, B, and C, EDR dose-dependently decreased cytokine production in the presence of LPS. Moreover, we examined whether EDR dose-dependently suppressed the mRNA and protein levels of the pro-inflammatory cytokines in LPS-stimulated cells by RT-PCR and western blot analysis (Fig. 3D and E) and again found that EDR decreased the LPS-induced cellular levels of IL-1β, IL-6, and TNF-α in a dose-dependent manner.
In order to determine the effect of EDR on anti-inflammatory cytokine production, IL-4 and IL-10 were measured by western blot in RAW 264.7 cells. Results demonstrated that IL-4 and IL-10 did not change significantly in a dose-dependent manner (Fig. 4). These results suggest that EDR did not affect IL-4 and IL-10 production at the protein level in activated macrophages.
To further evaluate whether EDR influences the phenotypic maturation of macrophages, the cells were cultured with EDR and LPS for 24 hrs and then analyzed surface expression of B7-1 and B7-2 molecules. As shown in Fig. 5, EDR slightly suppressed the expression of B7-1/-2 molecules.
Activation of the transcription factor NF-κB is required for the expression of various cytokines, iNOS, and COX-2 in macrophages in response to LPS. NF-κB is found in an inactive form associated with its inhibitor molecule (IκB) in the cytoplasm. Upon stimulation, rapid phosphorylation and degradation of IκBα allows NF-κB to translocate into the nucleus and regulate transcription of the target genes. We also studied the effect of EDR on NF-κB activation by western blot analysis of phosphorylation of IκBα in RAW 264.7 cells (Fig. 6). These results showed that EDR inhibits the LPS-induced NF-κB activation.
Anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) are usually indicated for the treatment of acute or chronic inflammation, but these drugs often have side effects. Researchers have performed screens to find new medicinal compounds from natural products that are inhibitors of inflammatory mediators with lower toxicity and that could be used in therapeutic application (18). Thus, in the present study, the anti-inflammatory activity of water extract of Dioscoreae Rhizome (EDR), which has been used for traditional medicine, on NO, cytokines, PGE2 and co-stimulatory molecules in LPS-stimulated murine macrophages was investigated.
Macrophages play key roles in immune responses, and their activation has been associated with a significant proportion of LPS-stimulated inflammation. Active macrophages produce inflammatory mediators, including reactive oxygen and nitrogen intermediates, hydrolytic enzymes, lipid mediators, and inflammatory cytokines (19).
Nitric oxide is a product of the enzymatic conversion of arginine to citrulline by a family of three distinct nitric oxide synthases (NOS). Expression of the inducible isoform of NOS (iNOS) and an increased concentration of NO may play a critical role in regulating physiological processes, such as blood vessel tone and neurotransmission, as well as in host defense and immunity (20,21). In this paper, we have demonstrated that EDR decreases NO production in RAW 264.7 cells (Fig. 1).
PGE2, which is produced by COX-2, plays important roles in endotoxemia and inflammatory conditions (22). In the prostaglandin biosynthesis pathway, COX-2 is primarily expressed in leukocytes and is the key enzyme in the conversion of arachidonic acid to PGE2. Therefore, COX-2 targeted therapy is under consideration for the treatment of inflammatory diseases. In the present study, EDR suppressed PGE2 production by inhibiting COX-2 activity and may be effectively involved in the regulation of the inflammation by this compound (Fig. 2).
Cytokines are regulated in almost all important biological processes such as cell activation, inflammation, immunity, and differentiation. Pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α are responsible for worsening inflammation, while anti-inflammatory cytokines such as IL-4 and IL-10 have marked inhibitory effects on the expression and release of pro-inflammatory cytokines. In this study, EDR inhibited the production of IL-1β, IL-6, and TNF-α through a decrease in the gene and protein levels of IL-1β, IL-6, and TNF-α in activated macrophages (Fig. 3); however, EDR did not exhibit significant dose-dependent effects on IL-4 and IL-10 protein expression levels (Fig. 4).
The transcription factor NF-κB is a ubiquitous protein that induces a variety of genes in the inflammation process (23,24). NF-κB binds κB motifs in the promoters via its p65 subunit, which is the inactive form (25). In the activation processes induced by LPS and other cytokines, NF-κB requires the phosphorylation of IκB for ubiquitination and degradation (26). In this report, we found that EDR reduced NF-κB translocation in LPS-induced RAW 264.7 cells by inhibiting IκBα phosphorylation (Fig. 6).
The B7 co-stimulatory molecules are expressed by various cell types involved in antigen presentation. Both B7-1 and B7-2 are found on activated antigen-presenting cells (APCs) and regulated during monocyte, macrophage and lymphocyte activation. Based on our results, treatment with EDR inhibits the co-stimulatory molecules, B7-1 and B7-2, in macrophages (Fig. 5).
In summary, the present study demonstrates that EDR exerts significant anti-inflammatory effects on LPS-induced macrophages by suppressing the production of inflammatory mediators, such as NO, PGE2, IL-1β, IL-6, and TNF-α. The inhibitory effects of EDR are associated with NF-κB inactivation and the reduction of IκBα phosphorylation. In addition, EDR also inhibits the expression of co-stimulatory molecules, including B7-1 and B7-2. Thus, our study provide the anti-inflammatory effects of Dioscoreae Rhizome and suggests that EDR might useful as a therapeutic drug for inflammatory diseases.
Figures and Tables
Figure 1
EDR inhibits the production of NO (A), expression of iNOS mRNA (B), and protein (C) in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in the absence or presence of LPS (100 ng/ml) overnight. Culture supernatants were then collected and NO concentrations were measured using Griess reagent (A). Cell lysates were extracted, and protein levels of iNOS were then analyzed by Western blotting (B). RAW 264.7 cells were incubated with EDR in the absence or presence of LPS for 24 h. Total RNA was isolated, and levels of iNOS mRNA were then measured by RT-PCR (C). Each value represents the mean±S.D. of three independent experiments. ††p<0.01 vs. cells only based on Student's t-test. **p<0.01 vs. LPS only based on Student's t-test.
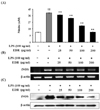
Figure 2
EDR inhibits the production of PGE2 (A), COX-2 mRNA (B) and protein (C) in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in the absence or presence of LPS (100 ng/ml) for 48 h. Culture supernatants were then collected and PGE2 concentrations were measured using ELISA kits (A). Cell lysates were extracted, and protein levels of COX-2 were then analyzed by Western blotting (B). RAW 264.7 cells were incubated with EDR in the absence or presence of LPS for 24 h. Total RNA was isolated, and levels of COX-2 mRNA were then measured by RT-PCR (C). Each value represents the mean±S.D. of three independent experiments. †p<0.01 vs. cells only based on Student's t-test.
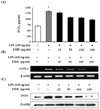
Figure 3
EDR inhibits pro-inflammatory cytokine production in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in the absence or presence of LPS (100 ng/ml) for 48 h. Culture supernatants were then collected and cytokine concentrations were measured using ELISA kits (A~C). RAW 264.7 cells were incubated with EDR in the absence or presence of LPS for 24 h. Cell lysates were extracted, and protein levels of each cytokine were then analyzed by Western blotting (D). Total RNA was isolated, and mRNA levels of each cytokine were then measured by RT-PCR (E). Each value represents the mean±S.D. of three independent experiments. ††p<0.01 vs. cells only based on Student's t-test. *p<0.05, **p<0.01 vs. LPS only based on Student's t-test.
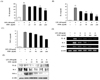
Figure 4
Effects of EDR on the anti-inflammatory cytokine production in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in the absence or presence of LPS (100 ng/ml) for 24 h. Cell lysates were extracted, and protein levels of each cytokine were then analyzed by Western blotting.

Figure 5
EDR inhibits the expression of co-stimulatory molecules in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were cultured and activated with LPS (100 ng/ml) in the absence or presence of various EDR concentrations for 24 hrs. The surface B7-1 (A) and B7-2 (B) were labeled with anti-B7-1/-2 antibodies and the cells were then stained using anti-Vβ8.1+8.2-FITC, anti-Vβ2-PE, or anti-Vβ2-FITC, which served as an isotype control for nonspecific binding.

Figure 6
EDR inhibits the IκBα phosphorylation in the cytoplasm and the nuclear translocation of NF-κB p65 in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with various concentrations (25, 50, 100, 200 µg/ml) of EDR in the absence or presence of LPS (100 ng/ml) for 24 hrs. Cell lysates were extracted, and protein levels of each cytokine were then analyzed by Western blotting.

References
1. Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003. 52:1799–1805.
2. Jacobs M, van Greevenbroek MM, van der Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, Jansen EH, Schalkwijk CG, Stehouwer CD. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: the CODAM study. Eur J Clin Invest. 2009. 39:437–444.

3. Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994. 91:2046–2050.

4. Lee TH, Kwak HB, Kim HH, Lee ZH, Chung DK, Baek NI, Kim J. Methanol extracts of Stewartia koreana inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) gene expression by blocking NF-kappaB transactivation in LPS-activated RAW 264.7 cells. Mol Cells. 2007. 23:398–404.
5. Nathan C. what difference does it make? J Clin Invest. 1997. 100:2417–2423.
7. Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol. 2005. 174:2825. 2833.

8. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003. 169:203. 214.

9. Tilg H, Wilmer A, Vogel W, Herold M, Nöolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992. 103:264–274.

10. Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998. 11:1218–1221.

11. Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994. 10:405. 455.
12. Renard P, Raes M. The proinflammatory transcription factor NFkappaB: a potential target for novel therapeutical strategies. Cell Biol Toxicol. 1999. 15:341–344.
13. Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res. 2003. 58:95–130.

14. Zhong X, Nishino E, Okagami N. Temperature dependence of seedling establishment of a perennial, Dioscorea tokoro. J Plant Res. 2002. 115:55–57.

15. Lee SC, Tsai CC, Chen JC, Lin CC, Hu ML, Lu S. The evaluation of reno- and hepatoprotective effects of huai-shan-yao (Rhizome Dioscoreae). Am J Chin Med. 2002. 30:609–616.

16. Tewtrakul S, Itharat A. Nitric oxide inhibitory substances from the rhizomes of Dioscorea membranacea. J Ethnopharmacol. 2007. 109:412. 416.

17. Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989. 169:1543. 1555.

18. Lee GI, Ha JY, Min KR, Nakagawa H, Tsurufuji S, Chang IM, Kim Y. Inhibitory effects of oriental herbal medicines on IL-8 induction in lipopolysaccha-rideactivated rat macrophages. Planta Med. 1995. 61:26–30.

19. Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995. 35:655–677.

20. Furchgott R, Cherry P, Zawadzki J, Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984. 6:S336–S343.

21. Wei X, Charles I, Smith A, Ure J, Feng G, Huang H. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995. 375:408–411.

22. Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002. 71:1005–1011.
23. Li Q, Verma IM. NFkappa B regulation in the immune system. Nat Rev Immunol. 2002. 2:725–734.
24. Richmond A. NF-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002. 2:664. 674.
25. Lee S, Shin S, Kim H, Han S, Kim K, Kwon J, Kwak JH, Lee CK, NJ Ha, Yim D, Kim K. Antipin-flammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. Inflammation. 2011. 8:16.
26. Karin M, Ben-Neriah Y. The control of NF-kappa B activity. Annu Rev Immunol. 2000. 18:621–663.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download