Abstract
Background
Vitamin C is an essential nutrient for maintaining human life. Vitamin C insufficiency in the plasma is closely related with the development of scurvy. However, in vivo kinetics of vitamin C regarding its storage and consumption is still largely unknown.
Methods
We used Gulo-/- mice, which cannot synthesize vitamin C like human. Vitamin C level in plasma and organs from Gulo-/- mice was examined, and it compared with the level of wild-type mice during 5 weeks.
Results
The significant weight loss of Gulo-/- mice was shown at 3 weeks after vitamin C withdrawal. However, there was no differences between wild-type and vitamin C-supplemented Gulo-/- mice (3.3 g/L in drinking water). The concentration of vitamin C in plasma and organs was significantly decreased at 1 week after vitamin C withdrawal. Vitamin C is preferentially deposited in adrenal gland, lymph node, lung, and brain. There were no significant changes in the numbers and CD4/CD8 ratio of splenocytes in Gulo-/- mice with vitamin C withdrawal for 4 weeks. And the architecture of spleen in Gulo-/- mice was disrupted at 5 weeks after vitamin C withdrawal.
Vitamin C is a co-factor of some enzymes such as dopamine-β-hydroxylase and collagen synthase which are essential for the life (1-3). That is to say, vitamin C insufficiency affects severe defects on cardiac function and skeletal systems due to the deficiency on the production of hormones and collagen (4,5). In addition, vitamin C plays an important role on the defense system against viral infection and the development of cancer (6-8). Even though there are still the arguments regarding the anti-viral and anti-tumor activity of vitamin C in vivo, lots of experiment in vitro showed that vitamin C is one of the effective nutrients for the prevention of tumor development and cancer therapy.
It is known that vitamin C (L-ascorbic acid) is synthesized from glucose, during the glycolytic pathway and L-gulonolactone-γ-oxidase (gulo) is one of the essential enzymes for the synthesis of vitamin C, especially conversion of L-gulonolactone to L-ascorbic acid (9,10). In the case of human being and some primates, a mutation of the gene encoded gulo is considered as the reason of the defect on the production of vitamin C (11). However, most of experimental animals could produce vitamin C by themselves, except guinea pig. Gulo-/- mice were generated and used for the investigation of the effect of vitamin C on the prevention of the formation of atherosclerotic plaque upon vitamin C insufficiency (12). Therefore, the limitation of in vivo experiments about the effect of vitamin C on the prevention or facilitation of development of diseases upon vitamin C supplementation or withdrawal has been overcome.
Regarding in vivo vitamin C pharmacokinetics, it was reported that plasma vitamin C concentration reaches the peak concentration at 2~3 hrs after administration (13). When vitamin C is administered via intravenous injection, its concentration in serum is 5~6 times higher than that of oral administration, and it is drastically decreased at 6 hrs after administration. According to the report by Harrison et al. (14), vitamin C is preferentially deposited in brain (4~10 mM), adrenal gland (2~10 mM), liver (0.8~1 mM) and cerebrospinal fluids (CSF; 0.2~0.4 mM). However, the reason why such organs contain the high concentration of vitamin C is still largely unknown. Moreover, in vivo kinetics of vitamin C in organs under vitamin C insufficient condition has not been investigated yet. Therefore, we examined the changes of vitamin C concentration in organs of Gulo-/- mice upon vitamin C withdrawal.
Gulo-/- mice were obtained from the Mutant Mouse Regional Resource Center (University of California, Davis, USA). C57BL/6 wild-type and the Gulo-/- mice were maintained in a specific pathogen-free condition at an animal facility in the Seoul National University College of Medicine. Male Gulo-/- mice (4~5 weeks old) were maintained for 5 weeks with or without vitamin C (3.3 g/L or 0.33 g/L, Sodium L-ascorbate, Sigma, St. Louis, MO, USA) supplementation in drinking water. The animal use protocol for the experiment (approved No. SNU-080624-3 and SNU-100428-3) was reviewed and approved by the Ethics Committee of the Seoul National University.
During the experimental periods for 5 weeks, plasma and organs were collected from wild-type and Gulo-/- mice with or without vitamin C supplementation at every week. Tissues were quickly frozen in liquid nitrogen, and then stored at -70℃ until use. After weighting, tissues were homogenized with TissueLyser II (Qiagen, Germany) in phosphate buffered saline (PBS). The homogenates were centrifuged with 14,000 rpm at 4℃ for 30 min, and the supernatants were used for vitamin C measurement.
Plasma and tissue homogenates were diluted in PBS. Vitamin C (ascorbic acid, AA) converted into dehydroascorbic acid (DHA), an oxidized form of ascorbic acid, and then the concentration of total DHA was measured by using a colorimetric microtiter plate assay kit (Immundiagnostik AG, Germany) according to the manufacturer's instructions. The final concentration of vitamin C in each tissue was normalized to tissue weight.
Spleen was removed and placed into cold washing media, which is RPMI media containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100µg/ml streptomycin (GIBCO, Carlsbad, CA, USA). Spleen was homogenized by passing through 70µm nylon mesh (BD Bioscience, San Jose, CA, USA) and centrifuged at 600 g for 10 min. The pellet was re-suspended in red blood cell lysis buffer (Sigma, St. Louis, MO, USA), and washed with washing media. The isolated splenocytes were stained with trypan blue (GIBCO, Carlsbad, CA, USA), and countered. Freshly isolated splenocytes were resuspended in FACS buffer containing 0.5% BSA and blocked at 4℃ for 10 min with Fc blocking reagent (Miltenyi Biotec GmbH, Germany). Then, cells were stained with anti-CD4 and anti-CD8 antibodies (BD Bioscience, San Jose, CA, USA) on ice for 30 min and washed twice with FACS buffer. Cells were analyzed by FACS Calibur (BD Bioscience, San Jose, CA, USA). FlowJo software (Tree Star, Ashland, OR, USA) was used for the data analysis.
Spleens were freshly excised, and fixed in 4% paraformaldehyde. The paraffin-embedded sections (5µm thickness) were deparaffinized with xylene and hydrated by alcohol series. Then, sections sere stained with hematoxylin and eosin (H&E, Sigma, St. Louis, MO, USA) according to the manufacturer's instructions. After mounting, stained sections were viewed with inverted light microscopy (Olympus, Center Valley, PA, USA).
Data were expressed as mean±S.D. of each group in independent experiments. For comparison of three or more groups, data were analyzed by one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test. A value of p<0.05 was considered to be statistically significant. Statistical tests were carried out using GraphPad InStat (GraphPad Software, San Diego, CA, USA).
We first investigated the phenotypic changes of Gulo-/- mice upon vitamin C withdrawal for 5 weeks. A skeletal change was observed from Gulo-/- mice without supplementation of vitamin C for 5 weeks (Fig. 1A). We found the decreased movement of Gulo-/- mice upon vitamin C withdrawal (data not shown). As shown in Fig. 1B, weight loss began at 3 weeks after vitamin C withdrawal and aggravated with weeks. We next compared the plasma concentration of vitamin C in four experimental groups; wild-type, Gulo-/- mice with vitamin C supplementation (3.3 g/L or 0.33 g/L), and Gulo-/- mice without vitamin C supplementation. There was no difference between wild-type and Gulo-/- mice supplemented with 3.3 g/L concentration of vitamin C. However, remarkable decrease of vitamin C concentration to sub-scurvy levels (less that 30µM) was observed at 1 week after vitamin C withdrawal and it lasted for 5 weeks (Fig. 1C). In addition, plasma levels of vitamin C in Gulo-/- mice supplemented with 0.33 g/L of vitamin C was compared with that of wild-type and Gulo-/- mice supplemented with 3.3 g/L of vitamin C. As a result, it levels in Gulo-/- mice supplemented with 0.33 g/L could not reach at the concentration in wild-type and Gulo-/- mice supplemented with 3.3 g/L of vitamin C. It suggests that the optimal concentration of vitamin C is 3.3 g/L for the examination of physiological effects of vitamin C in vivo.
In general, vitamin C uptake mainly occurred in gastrointestinal tracts via the vitamin C-specific transporters, sodium dependent vitamin C transporter (SVCT)-1 and -2 (15). Therefore, the concentration of vitamin C in stomach, large intestine and small intestine upon vitamin C withdrawal were examined. The concentration of vitamin C in large and small intestine was higher than stomach (Fig. 2A-C). In addition, it decreased at 1 week after vitamin C withdrawal and it lasted for 5 weeks. As we described, vitamin C is a derivative of glucose and liver is the site for glycolysis and gluconeogenesis (16). Therefore we also examined the concentration of vitamin C in liver. Relatively low concentration of vitamin C was deposited in liver (Fig. 2D). Like a plasma levels of vitamin C, the concentration of vitamin C in intestines, stomach and liver of Gulo-/- mice supplemented with 0.33 g/L concentration of vitamin C during the experimental periods was examined. It was similar with the concentration in those organs of Gulo-/- mice that is maintained without vitamin C for 1 week.
It is already known that vitamin C plays an important role in the collagen synthesis as a co-factor of collagen synthase (17). Therefore, it is considered that vitamin C has protective effect in brain and heart from the infarction through extensive vascular changing. For this reason, we examined the levels of vitamin C in brain and heart. As shown in Fig. 3A and B, high concentration of vitamin C was concentrated in both brain and heart. Interestingly, we found that the high concentration of vitamin C was maintained in brain until the end of experiments, approximately 1 mM at 5 weeks after vitamin C withdrawal. It seems that huge amounts of vitamin C are needed to protect brain from the damage by reactive oxygen species (ROS), since vitamin C is one of the well-known anti-oxidants. Based on the role of vitamin C as anti-oxidants, the concentration in lung was subjected to be analyzed. As we expected, high concentration of vitamin C is deposited as much as brain, but its concentration was more rapidly decreased (Fig. 3C). The concentration of vitamin C in brain, heart and lung of Gulo-/- mice supplemented with 0.33 g/L concentration of vitamin C was also relatively lower than in wild-type or Gulo-/- mice supplemented with 3.3 g/L of vitamin C.
Vitamin C is an essential factor for the production of hormones (17). So, we investigated the concentration of vitamin C in some organs related with the generation or action of hormones. Adrenal glands, which mainly produce corticosteroid hormones, contain vitamin C up to 5 mM (Fig. 4A). Even though it is also decreased at 1 week after vitamin C withdrawal, high concentration of vitamin C was maintained till 5 weeks after vitamin C withdrawal. Moreover, relatively low concentration of vitamin C was detected in pancreas, testis and kidney (Fig. 4B-D). The sudden fall of vitamin C concentration at 1 week after vitamin C withdrawal was also observed in adrenal gland, pancreas, testis and kidney like other organs. The concentration of vitamin C in these organs of Gulo-/- mice supplemented with 0.33 g/L of vitamin C was similar to that of Gulo-/- mice with vitamin C withdrawal during the experimental periods.
Since the most well-known function of vitamin C is immune potentiating, we finally examined the concentration of vitamin C in lymph node and spleen. Interestingly, we found that vitamin C is deposited at lymph node as much as adrenal glands (Fig. 5A). Even though the level was relatively low when it compared with the vitamin C concentration in lymph node, spleen contained high concentration of vitamin C like heart, large and small intestines (Fig. 5B). In the case of spleen, the alteration of splenic architecture in Gulo-/- mice by vitamin C withdrawal for 5 weeks was found (Fig. 5C). However, there were no significant differences in the splenocyte numbers and at the ratio between CD4 and CD8 T cell (Fig. 5D and E).
Vitamin C, glutathione, and vitamin E (α-tocopherol) are important members of intracellular anti-oxidant network and they protect organisms from the damages induced by oxygen free radicals, such as superoxide anion, nitric oxide and hydrogen peroxide. Even though it is known that anti-oxidant activity of vitamin C is less than other two molecules, it should be needed for the conversion of oxidized glutathione (GSSG) to reduced glutathione (GSH) and tocopheroxyl radical, an oxidized form of vitamin E, to α-tocopherol (4,5). It means that vitamin C is an essential factor for the maintenance of intracellular anti-oxidant network. Based on its anti-oxidant activity, we can suppose that vitamin C is preferentially accumulated in metabolically active organs, such as brain, lung and heart. In fact, it is reported that vitamin C uptake is mainly occurred at the endothelium of small intestine and preferentially deposited in brain (4~10 mM), adrenal gland (2~10 mM), liver (0.8~1 mM), muscle (0.4 mM) and cerebrospinal fluids (CSF; 0.2~0.4 mM), and 40~60µM of vitamin C is detected in serum and red blood cell (14). To understand physiological mechanisms related with the management of organs, in vivo vitamin C kinetic study regarding accumulation and its consumption in organs is strongly needed. Therefore, we examined in vivo vitamin C kinetic study upon vitamin C withdrawal by using Gulo-/- mice. This is the first reports regarding the storage and consumption of vitamin C in vivo using animals which cannot synthesize vitamin C like human.
Since Gulo-/- mice is unable to synthesize vitamin C in vivo like human, 0.33 g/L of vitamin C is recommended for their maintenance (www.mmrrc.org). However, we found remarkable decreasing vitamin C in plasma to the level of scurvy (0.33 g/L vitamin C-supplemented Gulo-/- mice: 24.88µM). When mice were maintained with 3.3 g/L, it was almost same as WT mice (WT: 88.41µM, 3.3 g/L vitamin C-supplemented Gulo-/- mice: 80.90µM). Most organ concentration of vitamin C in Gulo-/- mice supplemented with 0.33 g/L vitamin C was similar to the levels of Gulo-/- mice with vitamin C withdrawal for 1~2 weeks. It suggests that 0.33 g/L is minimum amounts of vitamin C only for the maintenance of Gulo-/- mice, but 3.3 g/L is optimal amounts for the investigation of the physiological functions of vitamin C in vivo. SMP30-/- mice are also used as an animal model for the analysis of vitamin C functions in vivo. For the maintenance of SMP30-/- mice, 1.5 g/L of vitamin C/day was used, because alternative pathway for vitamin C synthesis by using of D-glucurono-1, 4-lactone is intact (18). Therefore, Gulo-/- mice is maintained with 3.3 g/L concentration of vitamin C supplementation, and it is the most suitable experimental model for the assessment of in vivo vitamin C functions and its related mechanisms.
Vitamin C has a crucial role on the hydroxylation of proline, which is closely related to collagen synthesis, blood vessel formation, the synthesis of hormone and neurotransmitter and immune functions (19). Therefore, it seems that weight loss of Gulo-/- mice shown in Fig. 1B is caused by the defect of the hydroxylation of proline and collagen synthesis under vitamin C-insufficient condition. In relation with collagen synthesis, there are several reports regarding the important role of insulin-like growth factor (IGF) production (20,21). That is to say, severe defect of the synthesis of collagen was observed when IGF production is inhibited. Since we have previously reported that vitamin C inhibits the proliferation of human melanoma cell lines, SK-Mel2 via the suppression of IGF production (22), the defect of the hydroxylation of proline and collagen synthesis in Gulo-/- mice under vitamin C-insufficient condition should be further investigated. In addition, the decrease of food intake is regarded as the one of the reason for the weight loss of Gulo-/- mice. According to the report by Odumosu (23), vitamin C supplementation reduces weight loss of guinea pigs and its effect is synergistically up-regulated appetite when administered with serotonin.
Regarding the roles and functions of vitamin C on the anti-viral and anti-tumor immunity, it is believed that vitamin C directly increased the cytotoxic activity of natural killer (NK) cells or antigen specific cytolytic T cells (CTLs), but it is still controversial. It is because that most of experiments regarding the effect of anti-viral and anti-tumor immunity were done in vitro. Even if the experiments were done in vivo, it was impossible to distinguish whether the anti-viral and anti-tumor effects are induced by vitamin C supplementation, since most animals used in experiments can produce the large amounts of vitamin C by themselves. However, we found that the size of spleen was distinctively reduced and the structure of spleen was considerably disrupted in Gulo-/- mice by vitamin C withdrawal for 5 weeks. Therefore, it assumes that vitamin C is essential for the maintenance of the structure and functions of spleen. We also agree that vitamin C can directly increase of anti-viral and anti-tumor activity of NK cell and CTLs. Since we have previously reported that vitamin C directly induces apoptosis of tumor via the down-regulation of transferrin receptors and mitochondrial membrane potential bypassing the activation of NK cells and CTLs (24). The maintenance of the highest concentration of vitamin C via intravenous injection is useful for immunotherapy of cancer patient, since we can expect that vitamin C maintains effector functions of immune organs and immune cells as well as directly induces apoptosis of tumors.
Figures and Tables
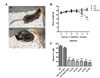 | Figure 1Loss of weight and decrease of plasma vitamin C concentration in Gulo-/- mice upon vitamin C withdrawal. (A) Phenotype of wild-type (WT) and Gulo-/- mice (KO) with vitamin C withdrawal for 5 weeks. (B) Changes of body weight upon vitamin C withdrawal were followed for 5 weeks (n=10). (C) The concentration of vitamin C of WT and Gulo-/- mice in plasma (n=4~10). ***p<0.001 vs. vitamin C- supplemented Gulo-/- mice (KO+VC, 3.3 g/L). |
 | Figure 2The changes of vitamin C concentration in gastrointestinal organs. The concentration of vitamin C of WT and Gulo-/- mice in (A) large intestine, (B) small intestine, (C) stomach and (D) liver (n=4~10). *p<0.05, **p<0.01, ***p <0.001 vs. vitamin C-supplemented Gulo-/- mice (KO+VC, 3.3 g/L). |
 | Figure 3The changes of vitamin C concentration in brain, heart and lung. The concentration of vitamin C of WT and Gulo-/- mice in (A) brain, (B) heart, and (C) lung (n=4~10). **p<0.01, ***p<0.001 vs. vitamin C-supplemented Gulo-/- mice (KO+VC, 3.3 g/L). |
 | Figure 4The changes of vitamin C concentration in adrenal gland, pancreas, testis and kidney. The concentration of vitamin C of WT and Gulo-/- mice in (A) adrenal gland, (B) pancreas, (C) testis and (D) kidney (n=4~10). **p<0.01, ***p<0.001 vs. vitamin C-supplemented Gulo-/- mice (KO+VC, 3.3 g/L). |
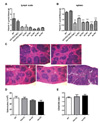 | Figure 5The changes of vitamin C concentration in lymph node and spleen, and the structural alteration in spleen. The concentration of vitamin C of WT and Gulo-/- mice in (A) lymph node and (B) spleen (n=4~10). ***p<0.001 vs. vitamin C-supplemented Gulo-/- mice (KO +VC, 3.3 g/L). (C) Splenic tissues from WT, Gulo-/- mice with vitamin C (3.3 g/L) supplementation and Gulo-/- mice with vitamin C withdrawal for 3~5 weeks were stained with H&E (×100). (D) The number of splenocytes and (E) the ratio of CD4 to CD8 T cells upon vitamin C supplementation and withdrawal. |
ACKNOWLEDGEMENTS
This work was supported by the grants from Seoul National University Hospital (Grant #0320110290) to Jae Seung Kang.
References
1. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999. 69:1086–1107.

2. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003. 22:18–35.

3. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989. 86:6377–6381.

4. Ellis GR, Anderson RA, Lang D, Blackman DJ, Morris RH, Morris-Thurgood J, McDowell IF, Jackson SK, Lewis MJ, Frenneaux MP. Neutrophil superoxide anion--generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J Am Coll Cardiol. 2000. 36:1474–1482.

5. Sauberlich HE. Packer L, Fuchs J, editors. A history of scurvy and vitamin C. Vitamin C in health and disease. 1997. New York: Marcel Dekker;1–24.
6. Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006. 50:85–94.

7. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976. 73:3685–3689.

8. Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008. 105:11105–11109.

9. Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem. 1992. 267:21967–21972.

10. Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994. 269:13685–13688.

11. Burns JJ. Missing step in man, monkey and guinea pig required for the biosynthesis of L-ascorbic acid. Nature. 1957. 180:553.

12. Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000. 97:841–846.

13. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996. 93:3704–3709.

14. Harrison FE, Green RJ, Dawes SM, May JM. Vitamin C distribution and retention in the mouse brain. Brain Res. 2010. 1348:181–186.

15. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999. 399:70–75.

16. Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007. 274:1–22.
17. Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986. 6:365–406.

18. Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol. 2010. 298:L784–L792.

19. Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006. 290:E1131–E1139.

20. Chojkier M, Spanheimer R, Peterkofsky B. Specifically decreased collagen biosynthesis in scurvy dissociated from an effect on proline hydroxylation and correlated with body weight loss. In vitro studies in guinea pig calvarial bones. J Clin Invest. 1983. 72:826–835.

21. Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991. 54:6 Suppl. 1135S–1140S.

22. Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008. 216:180–188.

23. Odumosu A. Anorectic drugs and vitamin C: role in appetite and brain ascorbic acid in guineapigs. Int J Vitam Nutr Res. 1981. 51:247–253.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download