INTRODUCTION
HMGB1 is one of the most abundant and highly conserved nonhistone chromosomal proteins in eukaryotes (1). HMGB1 interacts with several transcription factors, thereby allowing them to perform their cellular roles such as transcription, replication, and cellular differentiation (2). In addition to its roles within the nucleus, immune cells such as macrophages stimulated with LPS export nuclear HMGB1 to the cytoplasm and subsequently secrete it, and extracellular HMGB1 acts as an inflammatory mediator in various ways (2). It has been reported that HMGB1 facilitates diverse aspects of proinflammatory responses, including chemotaxis (3,4), increased permeability of cell monolayer (5), and the release of various proinflammatory cytokines such as TNF, IL-1, IL-6 and macrophage inflammatory protein-1 (6). Two recent papers demonstrated that HMGB1 can enhance proinflammatory activity by binding to LPS (7) and cytokines (8). These observations suggest HMGB1 can be involved in inflammation in direct and indirect manners.
In contrast to the clarity of the functions of HMGB1, the molecular mechanisms by which macrophages release HMGB1 are still unclear. Although many cytokines and signaling molecules are shown to be involved in this process (1) and modifications such as hyperacetylation and phosphorylation are needed for extracellular release (9), the relation and hierarchy between them are poorly understood. Recently, we have demonstrated that IFN-β plays a role in LPS-induced HMGB1 release as an intermediating molecule (10). However, we did not show the relation between IFN-β and other players involved in HMGB1 release.
In this paper, we show that CaMK, one of the mediators in HMGB1 release (11), exploits IFN-β pathway to regulate LPS-induced HMGB1 release. We also demonstrate that these signaling pathways are also needed in vivo. Our study suggests that the regulatory roles of CaMK in IFN-β production are indispensible for optimal release of HMGB1 in inflammatory conditions.
MATERIALS AND METHODS
Cells and reagents
Both mouse macrophage cell line, RAW 264.7 and human embryonic kidney cell line, 293 (American Type Culture Collection, Rockville, MD), were maintained in DMEM supplemented with 10% FBS and 0.1% penicillin/streptomycin (Life Technologies Korea, Seoul, Korea). LPS (O55:B5) were purchased from Sigma (St. Louise, MO). CaMK inhibitor, STO609, was obtained from Calbiochem (San Diego, CA). IFN-β was purchased from PBL Interferon Source (Piscataway, NJ). The following antibodies were used in this study: anti-HMGB1 (Abcam, Cambridge, MA), anti-phospho-IRF3 (Cell Signaling, Danvers, MA), anti-phospho-TANK-binding kinase 1 (TBK1) (BD Biosciences, San Jose, CA), anti-TBK1 (Cell signaling).
Mice
C57BL/6 (B6) mice were purchased from Daehan Biolink (Daejeon, Korea). All mice were housed under specific pathogen-free conditions at the animal facility of the Hallym University College of Medicine. Experiments were performed after the approval of the Animal Experimentation Committee at Hallym University (Hallym2009-57-1).
Measurement of cytokines
Concentrations of IFN-β were measured using ELISA kits (PBL Interferon Source) according to the manufacturer's instructions.
Real-time PCR
Total RNA from macrophages was extracted using Trizol reagents (Life Technologies Korea), according to the manufacturer's instruction and subjected to reverse transcription using Superscript II (Life Technologies Korea). Primers were purchased from Ambion Korea (Seoul, Korea) except TRIF (forward, AACCTCCACATCCCCTGTTTT; reverse, GCCCTGGCATGGATAACCA). Quantitative PCR was performed using SYBR green master mix (Qiagen Korea, Seoul, Korea). Genes were normalized to housekeeping gene, actin.
Western blot analysis
The level of HMGB1 was determined by western blotting. Samples of culture supernatant were concentrated with centricon (Milipore, Billerica, MA) and then separated on 12% SDS-PAGE gels, transferred to nitrocellulose membranes. The membranes were probed with anti-HMGB1 antibodies, and subsequently incubated with a HRP-conjugated secondary antibody. Bands were detected using SuperSignal West Femto kit (Thermo scientific, Rockford, IL). The amounts of HMGB1 were quantified by densitometric scanning of the exposed X-ray film. The relative densitometric value of HMGB1 of each sample shown as AU (arbitrary units) in the figures was calculated by arbitrarily assigning that of control (a LPS-treated sample) as 1 (10).
RESULTS
CaMK inhibitor, STO609 inhibits LPS-induced IFN-β production
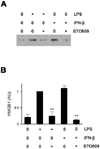
To investigate the mechanisms on LPS-induced HMGB1 release, we searched candidate molecules that seem to be involved in HMGB1 release. Among several candidates, we found that CaMK inhibitor, STO609, dramatically reduced LPS-induced HMGB1 release in RAW cells as previously reported (11). Since we previously demonstrated that IFN-β signal pathway is essential in HMGB1 release and IFN-β itself like LPS induces HMGB1 (10), we treated RAW cells with STO609 plus IFN-β and measured extracellular HMGB1. STO609 treatment reduced HMGB1 levels induced by IFN-β as well as LPS (Fig. 1), suggesting that STO609 could inhibit both up- and down-stream molecules of IFN-β. Since it was published that CaMKs are critical in type I IFN-induced JAK-STAT1 signaling (12), however, we turned our attention into upstream signals of IFN-β and investigated how CaMK is involved in LPS-induced IFN-β production. To test this, we treated RAW cells and primary macrophages with LPS plus various doses of STO609 and compared the levels of IFN-β using real-time PCR. STO609 reduced IFN-β production in a dose dependent manner in both cases (Fig. 2A and B). To dissect the action mechanism of STO609, we transfected 293 cells with Toll/IL-1 receptor domain-containing adaptor inducing interferon-β (TRIF) or TBK1 plasmids and treated them with STO609. TRIF or TBK1 transfected cells actively produced IFN-β mRNA without any stimulation. However, the transcription of IFN-β was dramatically reduced by STO609 in TRIF (Fig. 2C) or TBK1 (Fig. 2D) transfected cells.
Inhibition of CaMK blocks the phosphorylation of IRF3
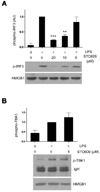
TLR4 uses two different adaptors, MyD88 and TRIF, and TRIF signals are relayed through TRIF-TBK1-IRF3 axis to produce IFN-β. In addition, our transfection assay suggested that STO609 does not affect TRIF-TBK1 themselves but their common effectors. These observations led us to test whether IRF3 is a target of STO609. RAW cells were treated with LPS plus various doses of STO609 and subjected to western blotting using anti-phospho-IRF3 antibody. Interestingly, STO609 reduced the phosphorylation of IRF3 in a dose dependent manner (Fig. 3A). Since the phosphorylation levels reflect the activity of IRF3, these data suggest that STO609 inhibits LPS-induced IRF3 function. Consistent with the above results, the phosphorylation of TBK1 was not reduced (Fig. 3B).
CaMKI is important in LPS-induced IFN-β production
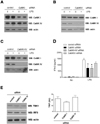
Since STO609, a selective inhibitor of CaMK kinase (13), can effectively block the activation of the downstream CaMKs (CaMKI and IV), we examined whether CaMKI and/or IV knock-down could also down-regulate IFN-β production. As shown in Fig. 4, endogenous CaMKI and IV expression in RAW cells was down-regulated by siRNA transfections (Fig. 4A~C). Compared with control siRNA, CaMKI siRNA significantly decreased LPS-induced production of IFN-β in RAW cells. Although CaMKIV siRNA also decreased IFN-β production, these inhibitory effects were not statistically significant. To test the synergistic effects of CaMKI and IV, we transfected RAW cells with both CaMKI and IV siRNAs simultaneously but did not find any synergistic effects (Fig. 4D). Next, we checked the expression levels of TRIF, TBK1, and IRF3 using CaMKI or IV knockdown cells and found no significant difference between control and knockdown cells (Fig. 4E).
CaMK signals are important to LPS-induced IFN-β release in vivo
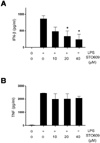
To evaluate the role of CaMKs in IFN-β production in vivo, we utilized the murine endotoxemia model. To test whether STO609 treatment reduces IFN-β levels, we administered LPS plus STO609 and measured blood IFN-β levels. Consistent with in vitro studies, the IFN-β levels of the STO609 treated mice were significantly lower than the control mice (Fig. 5A). However, the difference in mortality (data not shown) and blood TNF levels (Fig. 5B) were not seen. These mortality results seem to be a contrast to the previous reports that IFN-β deficiency improved the sepsis survivals (14,15). However, since a significant amount of IFN-β still remained in STO609 treated mice and the level of TNF is comparable to control mice (Fig. 5B), we assume that the protective effect of STO609 could be neglected in our experimental condition.
DISCUSSION
In this study, we have shown that CaMK regulates HMGB1 release via IFN-β and how CaMK is involved in IFN-β production. IFN-β synthesis was impaired in not only LPS treated macrophages but also TRIF or TBK1 transfected 293 cells by CaMK inhibitor, STO609 (Fig. 2). In addition, STO609 treatment reduced the levels of IRF3 but not TBK1 phosphorylation (Fig. 3) induced by LPS. These above findings suggest that the phosphorylation of IRF3 may be a target of CaMK. In knockdown study, we found that CaMKI played a major role (Fig. 4). To elucidate detailed interactions between CaMK isoforms and IRF3, we performed co-immunoprecipitation assay. However, we failed to detect any physical interaction between them (data not shown). This result can be interpreted in several ways. First, CaMK affects IRF3 activation in an indirect way. Second, the affinity between CaMK and IRF3 is relatively weak. And finally, other CaMK isoform like CaMKII (16) might be essential for IRF3 activation. Currently, we speculate that several CaMK isoforms and adaptors might form big complexes and induce IRF3 activation with low binding affinities as they do in the regulation of IFN-α-induced JAK-STAT activation (12).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download