Abstract
CD40-CD40L-mediated help from CD4 T cells is essential to induce primary CD8 T cell responses specific to the non-inflammatory cell-based antigen H60. In this study, using H60 as a model antigen, we generated recombinant vaccinia viruses (rVVs) expressing the H60 CD8 epitope and investigated whether CD4 help was required to activate the CD8 T cell response specific to the virally expressed H60. The immune response after infection with rVVs expressing H60 was similar to that after immunization with H60 congenic splenocytes, with a peak frequency of H60-specific CD8 T cells detected in the blood on day 10 post-infection. A CD8 T cell response specific for virally derived H60 was not induced in CD4-depleted mice, but was in CD40-deficient mice. These results provide insights into the characterization of the CD8 T cell response specifically for antigens originating from cellular sources compared to viral sources.
CD8 T cells play a major role in cell-mediated immune responses. They protect the body from pathogens and are also involved in the rejection of allogeneic transplants. These functions are performed by major histocompatibility complex (MHC) class I molecules that present peptides derived from pathogens or allogeneic antigens. The three phases of the CD8 T cell response following exposure to a foreign antigen are as follows: (a) clonal expansion of the activated T cells, (b) differentiation into effector cells, and (c) contraction of the expanded effector cells (1). Despite initial recognition by MHC-peptide complexes triggering T cell receptor (TCR)-mediated signals for CD8 T cells, it has been shown that help conferred by CD4 T cells is also essential in generating primary CD8 T cell responses after stimulation by non-inflammatory and cell-based antigens, such as antigens resulting from transplants and/or tumors (T helper cell [Th]-dependent) (2-4). The mechanism underlying CD4 T cell-mediated help with regard to CD8 T cells in response to stimulation by non-inflammatory antigens has not been clearly identified. However, previous studies have shown that induction of the CD8 T cell response is heavily dependent on the presence of activated CD4 T cells, which confers help via CD40/CD40L-mediated interactions between CD4 T cells and antigen presenting cells (APCs) (5-8).
It was initially thought that when CD8 T cells respond to infectious pathogens, CD4 help was not required for activating the primary response (Th-independent) due to the ability of the pathogen to activate APCs directly via Toll-like receptor (TLR)-mediated signaling (9,10). However, even during Th-independent CD8 T cell responses, CD4 T cell help was found to be essential for an optimal CD8 T cell response, including the generation of long-term memory cells after contraction of the effector cells (11-13).
Following an allogeneic transplantation, allogeneic minor histocompatibility antigens are presented by MHC molecules as peptide fragments derived from polymorphic regions of normal proteins (14). The CD4 and CD8 T cell responses to allogeneic minor histocompatibility antigens contribute to graft rejection or graft-versus-host disease (GVHD) in MHC-matched transplants between unrelated individuals. H60 is a minor histocompatibility antigen for which specific CD8 T cell response has been shown to be dominant in MHC-matched transplantation models (15,16). Its expression is restricted to hematopoietic cells (17). Because of these traits, the potential use of H60-specific CD8 T cells has been tried to model tumor treatments, such as the graft-versus-leukemia (GVL) effect (18). During the characterization of CD8 T cell responses specific to H60, we found that H60-specific CD8 T cells required CD4 help to induce the primary response and for expansion during the memory response (4,8). CD40/CD40L-mediated help is not only required for the primary response, but also for memory expansion of H60-specific cells. In addition, this CD4 help influences the diversity of CD8 T cells recruited to the response (19), supporting the hypothesis that CD8 T cell responses to a cell-based antigen are dependent on CD4 help. Based on these results, we investigated whether the CD8 T cell response to a virus-derived H60 CD8 epitope would be dependent on CD4 help.
Vaccinia virus (part of the poxvirus family) has been used as a vaccination vehicle, as it induces a strong CD8 T cell response in Balb/c and C57BL/6 mice (20,21). And we also generated recombinant virus expressing H60 CD8 eptiope for use in our study, based on the fact that recombinant vaccinia viruses expressing various model antigens, such as ovalbumin (22) hemagglutinin (23), and Gp33 from lymphocytic choriomeningitis virus (LCMV) (24), have been generated and used previously in other studies for antigen processing and presentation (22,25) and in the characterization of specific CD8 T cell responses (23,26,27).
In this study, we generated two different types of vaccinia virus: rVV-H60 (expressing the H60 CD8 epitope only) and rVV-H60+HY (expressing an additional CD4 epitope derived from the HY-Dby gene). We used these to investigate the need for CD4 T cell help in inducing primary CD8 T cell responses specific for H60 derived from viral sources. Our results show that primary CD8 T cell responses require CD4 T cell help, but they do not need CD40. These results provide information regarding the control of CD8 T cell responses to the natural minor histocompatibility antigen H60.
Recombinant vaccinia viruses were generated using a pSC11 vector (a kind gift from Dr. J.C. Yewdell, NIAID, Bethesda, MD, USA). These were comprised of a minigene corresponding to a region from the N terminal to the CD8 epitope sequence (39~46 amino acids [aa]) that was cloned into the NotI site of a pSC11 vector to generate rVV-H6039. To generate rVV-H6039+HY-Dby608, a minigene containing sequences corresponding to the CD4 epitope of HY-Dby (608~622 aa) with an additional Met at the N-terminal was cloned into the rVV-H6039 vector. Next, 143B cells were transfected with the plasmid described above and TK recombinants were isolated by plaque assay (25). Virus stock was produced in CV-1 cells and the viral titer was determined using BSC-1 cells.
C57BL/6 (B6) mice and H60 congenic mice (B6.CH60) were maintained under specific pathogen free (SPF) conditions at the Center for Animal Resource Development, Seoul National University College of Medicine. CD40-deficient mouse was purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained under SPF conditions at the Center for Animal Resource Development Seoul National University College of Medicine.
Female B6 mice were infected either by intraperitoneal (i.p.) injections with different titers of recombinant vaccinia virus or immunized by i.p. injection with H60 congenic splenocytes (2×107). The immunization studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Seoul National University.
B6 mice immunized either with rVVs expressing H60 or H60 congenic splenocytes were periodically bled retro-orbitally. Peripheral blood lymphocytes (PBLs) were prepared by red blood cell (RBC) lysis and stained with the following: phycoerythrin (PE)-conjugated H60 tetramer, allophycocyanin (APC)-conjugated anti-CD8 (53-6.7; eBioscience, San Diego, CA, USA), and fluorescein isothiocyanate (FITC)-conjugated anti-CD11a (M174; eBioscience) monoclonal antibodies (mAbs). The PBLs were then placed in fluorescence-activated cell sorter (FACS) buffer (1×phosphate-buffered saline [PBS] with 0.1% bovine calf serum, 0.05% sodium azide) at 4℃ for 30 min. After being washed with FACS buffer, cells were analyzed using a FACSCalibur flow cytometer equipped with Cell Quest software (BD Pharmingen, San Diego, CA, USA).
In order to virally express the H60 epitope, two recombinant vaccinia viruses were constructed (rVV-H6039 and rVV-H6039+HY-Dby608). They expressed the H60 determinant under the control of the viral p7.5 early/late promoter and were constructed by cloning the corresponding sequences into a modified pSC11 vector (25). rVV-H6039 expressed H60 from the N-terminal leader peptide to the CD8 epitope sequences (H6039-46), and rVV-H6039+HY-Dby608 expressed the HY-Dby CD4 epitope sequence as well as the H60 epitope (Fig. 1A). Following infection with rVV-H6039, it was expected that CD8 T cells reactive to H60 would arise with help from CD4 T cells which would respond to the CD4 epitopes derived from intrinsic viral proteins (28). Infection with rVV-H6039+HY-Dby608 was expected to generate HY-Dby-specific CD4 T cells that would help the H60-reactive CD8 T cells. This was performed in order to identify the specificity of CD4 T cells activated following infection.
We compared the ability of the two different types of rVVs expressing H60 to induce specific CD8 T cell responses. Female B6 mice were i.p. injected with varying doses of the virus (from 0.8×106 PFU to 8×106 PFU). We ascertained the number of H60-specific CD8 T cells in the blood of injected mice by staining PBLs with an H60-tetramer and subsequently performing the flow cytometry analysis. The results from the flow cytometry analysis showed that there were more H60 tetramer-binding CD8 T cells in the blood of B6 mice infected with rVV-H6039+HY-Dby608 than those infected with rVV-H6039 (Fig. 1B). We found between 7~13% of H60 tetramer-binding CD8 T cells in the blood of mice infected with 8×105 to 3.2×106 PFU of rVV-H6039+HY-Dby608. However, the frequencies ranged between 2~4% of peripheral blood CD8 T cells in mice infected with rVV-H6039. Infection with a higher titer (8×106 PFU) of rVV-H6039 did not increase the number of H60 tetramer-binding CD8 T cells in the blood. These results confirm that infection with both types of virus can induce the CD8 T cell response specific to a virally-originated H60 determinant and demonstrate that CD4 T cells are activated by recognition of virally-expressed HY-Dby CD4 determinant. Despite the fact that the vaccinia virus itself expresses several CD4 target epitopes that originate from the intrinsic viral proteins (28), the results suggest that the HY-Dby CD4 determinant expressed on the recombinant virus might be effective in providing help, perhaps due to the expression of the CD4 determinant as a processed oligopeptide.
We subsequently investigated the kinetics of the immune response induced by infection with rVV-H6039+HY-Dby608. Eight female B6 mice were i.p. injected with rVV-H6039+HY-Dby608 (1×106 PFU) and periodic retro-orbital blood sample were obtained. The PBLs were stained with the H60-tetramer and subjected to flow cytometry analysis. In order to ascertain whether the infection could generate an H60-specific CD8 T cell response at a comparable level to that induced by H60 from cellular sources, female B6 mice were i.p. immunized with splenocytes from H60 congenic mice. The resulting frequency plot of H60-tetramer-binding cells in peripheral blood CD8 T cells (shown against time) resulted in a kinetic curve similar between the mice that were infected with rVV-H6039+HY-Dby608 and were immunized with H60 congenic splenocytes (Fig. 2). Responses peaked on days 10~14 post immunization and waned afterwards in both cases. Even though the peak values were slightly higher in the mice infected with rVV-H6039+HY-Dby608 than those immunized with H60 congenic splenocytes (an average of 13% of peripheral blood CD8 T cells compared to an average of 7.5%, respectively), the peak values fell within a normal range (4). This result demonstrates that the immune response induced after infection with rVVs expressing the H60 epitope induced a CD8 T cell response comprised of an initial burst of cell expansion followed by a contraction phase (10).
As the primary CD8 T cell response which is specific for H60 originating from cellular sources is dependent on CD4 T cell help (4,8), we wanted to ascertain whether CD4 T cell help is also needed to induce primary CD8 T cell response specific for a virally-derived H60 epitope. We induced a primary CD8 T cell response to H60 in female B6 mice that were depleted of CD4 T cells following treatment with GK1.5 (an anti-CD4 mAb-depleting GK1.5). This was done by infecting the mice with rVV-H6039+HY-Dby608 and analyzing the PBLs periodically via flow cytometry in order to detect any increase in H60 tetramer-binding CD8 T cells. Mice were treated with Rat IgG and infected with rVV-H6039+HY-Dby608 as a control. After staining the PBLs (sampled periodically) from the two groups of infected mice, we found that there were very few H60 tetramer-binding cells in the blood of depleted and infected mice during the examination period (days 4 to 28 post-infection). However, there was a significant expansion of H60 tetramer-binding cells in the non-depleted and infected mice between days 7 to 10 (Fig. 3). Based on these results, we conclude that the presence of CD4 T cells is essential for the induction of primary CD8 T cell expansion in response to an infection with rVV-H6039+HY-Dby608. This result is consistent with previous findings which show that CD4 T cells are required for a primary CD8 T cell response to infection with recombinant virus encoding hemagglutinin (HA) (23). The results also indicate that CD4 help is needed for the induction of a CD8 T cell response to H60 originated from a vaccinia virus.
It has been shown that help from CD4 T cells is mediated via a CD40/CD40L interaction between CD4 T cells and APCs. The APCs are thought to stimulate the CD8 T cells by presenting peptide MHC I complexes (5-7). We have previously reported the significant roles of CD40 and CD40L in the induction of the CD8 T cell response after immunization with H60 congenic splenocytes (4,8). As the CD8 T cell response to cellular H60 and viral H60 are both CD4 T cell-dependent, we questioned whether the induction of H60-specific CD8 T cell responses after infection with rVV-H6039+HY-Dby608 would be impaired in CD40-deficient mice, as was the induction of CD8 T cell response by immunization with H60 congenic splenocytes. In order to answer this question, we challenged CD40-deficient mice and WT B6 mice with rVV-H6039+HY-Dby608 and analyzed PBLs periodically to detect H60-tetramer-binding CD8 T cells in the blood. We also immunized mice with H60 congenic splenocytes as a control to test for help-dependent on the CD40-CD40L interaction. The results from the flow cytometry analysis after staining the PBLs with an H60-tetramer showed that a similar expansion of H60-specific CD8 T cells was induced in the both CD40-deficient and WT B6 mice (Fig. 4). The peak frequencies of the H60-tetramer binding cells were 8~10% of peripheral blood CD8 T cells in the CD40-deficient mice infected with rVV-H6039+HY-Dby608 and 7~13% in infected WT B6 mice. This finding is in contrast to results from immunization of CD40-deficient mice with H60 congenic splenocytes, where no H60 tetramer-binding cells were detected in the blood. These results show that even though CD4 help is essential for the induction of a CD8 T cell response specific for virally derived H60, CD40 is not an essential factor for this to occur. This suggests that the CD4 help required for the induction of a primary CD8 T cell response might operate differently, depending on whether the antigenic source is cell-based or viral. It has been reported that CD8 T cell responses induced after infection by adenovirus are dependent on CD4 help (29), but not on CD40 (30). It has been shown that CD70-mediated APCs play a significant role in CD8 T cell responses to the ovalbumin adenoviral antigen, in the absence of CD40 (30). It is therefore possible that CD8 T cells enlist CD4 help only for virally expressed H60, and that this response might be mediated by CD70 expressed on dendritic cells in the absence of any CD40-CD40 interaction.
In summary, we report on the generation of a recombinant vaccinia virus expressing an H60 CD8 epitope, as well as on the fact that CD8 T cells require help to react to virally originated H60. We show that the induction of the primary CD8 T cell response specific for H60 after rVV infection has not been impaired in CD40-deficient mice. The results obtained from this study will further our understanding and characterization of CD8 T cell responses specific for antigens derived from cells compared to those derived from pathogens.
Figures and Tables
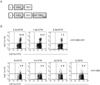 | Figure 1Generation of recombinant vaccinia viruses expressing the H60 CD8 epitope. (A) To generate a recombinant vaccinia virus expressing the H60 CD8 epitope (rVV-H6039), a minigene corresponding to a region encompassing a leader signal peptide to the epitope sequences (39~46 amino acids) of H60 was cloned into a pIRES vector. The DNA fragment encompassing the leader signal to the IRES region containing a multi-cloning site was cloned into a pSC11 vector. rVV-H6039+HY-Dby608 expressed the HY-Dby CD4 epitope peptide (608~622 aa) with an additional Met at the N-terminal, as well as the H60 CD8 epitope. This was achieved by cloning the corresponding minigene into the cloning site located after the IRES region of the rVV-H6039-pSC11 vector. (B) Female B6 mice were immunized with different amounts of rVV-H6039+HY-Dby608 and rVV-H6039 in order to determine the optimal titer of virus needed for the induction of H60-specific CD8 T cell responses, and to compare the ability of each virus to induce the specific immune response. Day 7 post-infection PBLs from immunized mice were stained with H60-Tet-PE, CD11a-FITC, and CD8-APC and analyzed by flow cytometry. Data from the flow cytometry analysis are shown here after gating on CD8+ cells. |
 | Figure 2Kinetics of the immune response after infection with rVV-H6039+HY-Dby608. Female B6 mice were immunized with rVV-H6039+HY-Dby608 or male H60 congenic splenocytes and periodic retro-orbital blood samples were obtained. PBLs were stained with H60-Tet-PE, CD11a-FITC, and CD8-APC for flow cytometry. The frequencies of H60-tetramer binding cells in the peripheral blood CD8 T cells at each time point are plotted as kinetics curves. Representative data from flow cytometry performed on day 10 post-immunization are shown after gating on CD8+ cells. These data were obtained from two independent experiments. |
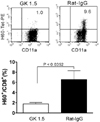 | Figure 3Dependency of the CD8 T cell response to a virally expressed H60 epitope on CD4 help. Female B6 mice were treated with GK1.5 (anti-CD4 mAb) or Rat-IgG (control) three days prior to infection with rVV-H6039+HY-Dby608. PBLs taken periodically from the two different immunized groups were stained with H60-Tet-PE, CD11a-FITC, and CD8-APC. Representative flow cytometry data obtained on day 10 post-infection (peak point) are shown. The peak frequencies observed from four individual mice in each group are plotted. These data were obtained from two independent experiments. |
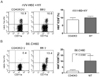 | Figure 4The CD8 T cell response specific for virally expressed H60 is independent of CD40. This figure shows female CD40-deficient and wild type B6 mice immunized with rVV-H6039+HY-Dby608 (A) and female CD40-deficient and wild type B6 mice immunized with male H60 congenic splenocytes (B). PBLs taken periodically from immunized mice were stained with H60-Tet-PE, CD11a-FITC, and CD8-APC. Representative flow cytometry data obtained on day 10 post-infection (peak point) are shown. The peak frequencies observed from five individual mice in each group are plotted. These data were obtained from two independent experiments. |
ACKNOWLEDGMENTS
This study was supported by a grant from the National Research Foundation of Korea (2009-0079925).
References
1. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002. 2:251–262.

2. Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982. 155:768–782.

3. Rees MA, Rosenberg AS, Munitz TI, Singer A. In vivo induction of antigen-specific transplantation tolerance to Qa1a by exposure to alloantigen in the absence of T-cell help. Proc Natl Acad Sci U S A. 1990. 87:2765–2769.
4. Ryu SJ, Jung KM, Yoo HS, Kim TW, Kim S, Chang J, Choi EY. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009. 113:4273–4280.
5. Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998. 393:478–480.

6. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998. 393:480–483.

7. Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998. 393:474–478.

8. Jung KM, Choi EY. Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60. Immune Netw. 2007. 7:173–178.

9. Rahemtulla A, Fung-Leung WP, Schilham MW, Kündig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, Miller RG. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991. 353:180–184.

11. Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003. 421:852–856.

12. Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003. 300:337–339.

13. Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003. 300:339–342.

14. Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002. 190:86–94.

15. Choi EY, Yoshimura Y, Christianson GJ, Sproule TJ, Malarkannan S, Shastri N, Joyce S, Roopenian DC. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: the H60 histocompatibility antigen dominates over all others. J Immunol. 2001. 166:4370–4379.

16. Choi EY, Christianson GJ, Yoshimura Y, Sproule TJ, Jung N, Joyce S, Roopenian DC. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002. 17:593–603.

17. Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, Shastri N. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998. 161:3501–3509.

18. Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, Gooley TA, Brown ML, Koo KK, Rosinski KV, Ogawa S, Matsubara A, Appelbaum FR, Riddell SR. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010. 115:3869–3878.

19. Choi JH, Ryu SJ, Jung KM, Kim S, Chang J, Kim TW, Choi EY. TCR diversity of H60-specific CD8 T cells during the response evolution and influence of CD4 help. Transplantation. 2009. 87:1609–1616.
20. Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002. 76:3329–3337.
21. Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004. 172:6265–6271.

22. Norbury CC, Princiotta MF, Bacik I, Brutkiewicz RR, Wood P, Elliott T, Bennink JR, Yewdell JW. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J Immunol. 2001. 166:4355–4362.

23. Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007. 179:8243–8251.

24. Agnellini P, Wiesel M, Schwarz K, Wolint P, Bachmann MF, Oxenius A. Kinetic and mechanistic requirements for helping CD8 T cells. J Immunol. 2008. 180:1517–1525.

25. Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994. 152:381–387.

26. Hwang ML, Lukens JR, Bullock TN. Cognate memory CD4+ T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8+ T cells and enhance tumor control. J Immunol. 2007. 179:5829–5838.

27. Xiao Z, Curtsinger JM, Prlic M, Jameson SC, Mescher MF. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of functional responses. Int Immunol. 2007. 19:733–743.

28. Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, Pasquetto V, Crotty S, Croft M, Lefkowitz EJ, Grey H, Sette A. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J Immunol. 2007. 178:6814–6820.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download