INTRODUCTION
Nanotechnology is one of the exponentially developing technologies of the 21st century. Nanoparticles sized between 1 and 100 nm are already in use for the most part including cosmetics, food industry, medicine, electronics, etc (1). However, because nanoparticles have large surface areas, high chemical reactivity, internal pore volumes, and enhanced cell penetrability, it may induce much more toxic effects (2-5).
Silica (SiO2) is one of the most abundant compounds found in nature as crystalline or amorphous silica (6). Silica has been considered an ideal nanoparticle for biomedical applications such as gene therapy, drug delivery, and biomedical imaging (7). However, it is becoming apparent that silica has cytotoxic and genotoxic effects. In human lymphoblastoid cells, ultrafine crystalline silica inhibited cell viability and growth, and also induced apoptosis and DNA strand breaks of cells (8). Ultrafine silica induced oxidative stress and pro-inflammatory responses in macrophages, mice and rats (6,9,10). Also, pulmonary inflammation, emphysema, alveolar hyperinflation, and apoptosis of alveolar and granulomatous cells have been found in animals exposed to silica (11,12). According to several toxicity results, silica has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) in 1997 (6).
Dendritic cells (DCs) are potent antigen-presenting cells, which reside in most tissues including blood and lymphoid organ (13-15). They function as sentinels of immune system and initiators of innate and adaptive immune responses. Mature DCs that have recognized antigen in peripheral tissue migrate to secondary lymphoid tissues and present the antigen to naïve T cells. In consequence, T cell responses are initiated (16,17). In mice, CD11c has been acknowledged as a typical DC marker. When DCs mature, they express high levels of MHC molecules, CD54, CD80, CD86, etc (18). Through expression of co-stimulatory molecules and cytokine secretion such as IL-12, IL-10, and IL-23, DCs induce activation of naïve T cells (15).
Vallhov et al. have reported size-dependent effects of mesoporous silica nano- (270 nm) and microparticles (2.5 µm) on human dendritic cells (5). The viability of monocyte-derived DC was inhibited by mesoporous silica. Also, the mesoporous silica induced immune regulatory signals through an alteration of co-stimulatory molecule expression and production of IL-12p70. Of the plentiful nanoparticles available, those sized 2~50 nm are of special interest in biotechnologies. Therefore, in the present study, we evaluated whether ultrafine (20~50 nm) silica induces a cytotoxic effect and inflammation in mouse dendritic cells. Ultrafine silica decreased the viability of DCs and increased the amount of cell deaths. Moreover, we have found that it has a differential effect on surface molecules on DCs after exposing them to silica in different frequency and concentration. In addition to the effect on DC differentiation, it induced TNF-α production in dendritic cells and led to inflammatory responses in vitro and in vivo, suggesting that ultrafine silica nanoparticles have a cytotoxic effect on dendritic cells and an immune modulation effect.
MATERIALS AND METHODS
Mice and cell lines
C57BL/6 mice were purchased from Samtako (Osan, Republic of Korea). Mice were maintained in specific pathogen-free conditions and used at 5~7 weeks. The experiments employing the mice were performed in accordance with institutional guidelines. The DC2.4 cell line, which was established as a murine dendritic cell line, was kindly provided by Dr. K. Rock of Harvard Medical School (19).
Preparation of silica nanoparticles and cell culture
All silica nanoparticles were purchased from Sigma-Aldrich (St. Louis, MO). Transmission electron microscopy (TEM) image of the same nanoparticles has already been reported elsewhere (6). Silica nanoparticles were suspended in distilled water and were autoclaved to inactivate any contaminating endotoxin. Dendritic cells and DC2.4 cells were cultured in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (HyClone, Logan, UT). Growth factors used in the primary culture of DCs were recombinant mouse GM-CSF and IL-4 (R&D Systems, Minneapolis, MN). FITC-conjugated antibodies to CD11c, CD54, CD80, and PE-conjugated antibodies to CD86, and MHC class II were purchased from BD Pharmingen (Palo Alto, CA).
Dendritic cell preparation from bone marrow
To obtain bone marrow-derived DCs, we used a method by Inaba et al. (20). Briefly, bone marrow cells isolated from femurs of C57BL/6 mice were harvested and incubated for 30 min at 4℃ with an antibody cocktail containing seven monoclonal antibodies, designated RA3-3A1/6.1, J11d.2, J1J.10, GK1.5, M5/114.15.2, F4/80, and 3.168. The cells were washed with culture medium and treated with rabbit complement (Low-ToxR-M, Cedarlane, Ontario, Canada) according to the manufacturer's instruction. Viable cells were then isolated by a density gradient centrifugation on Histopaque 1077 (Sigma-Aldrich) and washed twice with culture medium devoid of serum. The lymphocyte-depleted bone marrow cells were distributed in 24-well plates at 5-10×105 cells/ml. The cells were incubated in RPMI 1640 medium, supplemented with 10% heat-inactivated FBS (Hyclone). The media were supplemented with mouse GM-CSF (10 ng/ml) and IL-4 (10 ng/ml). Every 2 days, culture media were replaced. On days 6 or 7, the nonadherent cells were harvested by a gentle swirling and used in subsequent experiments.
MTT assay
DC 2.4 cells were seeded at a density of 1×104 cells/ml in 96-well plates and incubated with RPMI-1640 medium containing 10% FBS in the presence of various concentrations and sizes of silica nanoparticles. After 24 h incubation, 0.5 mg/ml of MTT solution was added to each well. After incubation for 3~4 h at 37℃, the MTT solution was removed. The incorporated formazan crystals in viable cells were solubilized with 100 µl of dimethyl sulfoxide. The absorbance was determined using VICTOR3™ (PerkinElmer, Waltham, MA).
Trypan blue exclusion assay
To analyze the growth in serum-containing medium, the cells were plated in a 100 mm dish in RPMI 1640 medium containing 10% FBS in the presence of various concentrations and sizes of silica nanoparticles. Cells were harvested after 24 and 48 h and then stained with 0.4% trypan blue and counted using a hematocytometer.
Analysis of cell death by annexin V and 7-AAD Staining
Cells cultured on culture dishes with silica nanoparticles for 24 h were harvested and stained with annexin V (BD Biosciences, Bedford, MA) and 7-AAD (BD Biosciences). The cell death was analyzed by flow cytometry using FACSCanto™II (BD Biosciences).
Flow cytometry
DCs produced by in vitro culturing were subjected to flow cytometric analysis using a FACSCanto™II. Cells were allowed to react with appropriate antibodies against CD11c, CD54, CD80, CD86, and MHC class II at 4℃ for 30 min and analyzed for their antigen expression.
RT-PCR
RNA was isolated with Trizol (Gibco/Invitrogen). The first strand cDNA was synthesized from 1 µg of total RNA using M-MLV reverse transcriptase (Promega, Madison, WI). β-actin was used as a loading control. PCR products were electrophoresed on a 1% agarose gel and visualized by ethidium bromide staining.
Matrigel plug assay
Silica nanoparticles, cell supernatants, or silica nanoparticle-treated DC2.4 cells were mixed with Growth Factor Reduced Matrigel™ Matrix (BD Biosciences). The Matrigels (700 µl each) were injected subcutaneously into the abdominal region of C57BL/6. After 9~11 days, mice were euthanized and matrigel plugs were removed. To evaluate hemoglobin level in matrigel plug, the Drabkin's reagent kit (Sigma) was used. The plugs were homogenized in 0.5 ml of distilled water and it was dissolved at 4℃. After then, homogenates were centrifuged and solution separated from homogenates was incubated with 0.5 ml of Drabkin's solution for 15 min at room temperature. Absorbance values were measured at 540 nm.
H&E staining
Silica nanoparticle-treated DC2.4-matrigel plugs were fixed with 10% formaldehyde overnight at 4℃. Samples were dehydrated, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E).
Western blot analysis
DC2.4 cells were exposed to silica nanoparticles (40 µg/ml) for 6 h. Cells were washed with DPBS and lysed in protein extraction solution (iNtRON Biotechnology, Seongnam, Republic of Korea). Proteins were separated on a 12% SDS-polyacrylamide gel and blotted onto a PVDF membrane, which was then blocked by incubating with TBST (Tris-buffered saline and 0.05% Tween-20) containing 5% skim milk. Membranes were incubated with specific antibodies and washed with TBST. All antibodies for MAPK were purchased from Cell Signaling Technology Inc. (Beverly, MA) and antibodies for IκB-α and actin were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Antigen-antibody complexes were visualized after incubating the membrane with diluted secondary antibody coupled to horseradish peroxidase and detected by enhanced chemiluminescence.
RESULTS
Cytotoxic effects of silica nanoparticles
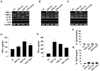
We evaluated cytotoxic effects of silica nanoparticles on murine dendritic cell line, DC2.4. The names and the sizes of silica nanoparticles used are as follows: SNP-20, 10~20 nm; SNP-50, 50 nm; SNP-5000, 1~5 µm. We performed MTT assay and trypan blue exclusion assay to estimate cell proliferation and viability. DC2.4 cells were cultured with silicas of different concentrations and sizes for 24 or 48 h. Ultrafine silica nanoparticles significantly decreased cell viability, depending on size and concentration (Fig. 1A). Because silica nanoparticles can interfere with MTT assay (21,22), trypan blue exclusion assay was also performed. Similar to the preceding MTT assay, as the size and the concentration of ultrafine silicas increased, the proliferation of DC2.4 cells was reduced (Fig. 1B). However, 1~5 µm sized silica particles had little toxic effect compared to ultrafine silica nanoparticles (Fig. 1A and B).
The cell death induced by ultrafine silicas was evaluated by measuring the binding of 7-amino-actinomycin D (7-AAD) and annexin V. As shown in Fig. 1C and D, DC2.4 cells showed an increase in cell death after being exposed to silica for 24 h. From these data, it is obvious that ultrafine silica nanoparticles have cytotoxic effect on dendritic cells.
Effect of silica nanoparticles on DC differentiation
In order to examine the phenotypic changes of bone marrow-derived DCs (BMDC) following exposure to silicas, we added silicas along with GM-CSF and IL-4 during DC differentiation or added for 24 h after the complete DC differentiation. In case of exposure to silicas after DC differentiation, immature DCs were co-cultured with 40 µg/ml of silica for 24 h. In case of exposure to silicas during DC differentiation, cells were co-cultured with 10 µg/ml of silica every 2 days, repeatedly. In DCs exposed to silicas after differentiation, expression of CD11c was inhibited by silicas which have a size of 10~20 nm and above (Fig. 2A). The expression of CD86 and MHC class II slightly increased after exposure to all ranges of particle size. There was no meaningful effect on the expression of CD80. In DCs exposed to silicas during differentiation, not only the expression of CD11c, but also the expression of all co-stimulatory molecules and MHC class II were reduced by silicas (Fig. 2B). Thus, DCs with different differentiation status exposed to silicas induced dissimilar changes of surface molecule expression. However, our findings showed that the effects of silica particles on the expression of DC surface molecules did not occur in nanoparticle-specific manner.
Effect of silica nanoparticles on cytokine production in DC
We further investigated whether silica nanoparticles are able to affect inflammatory cytokine production at transcriptional and translational levels in the dendritic cell line DC2.4 and bone marrow-derived DCs. Just like the preceding experiments, the cells were exposed to silicas together with GM-CSF and IL-4 during DC differentiation or exposed to silicas only for 24 h after the complete DC differentiation. The supernatants of dendritic cells were obtained from supernatants during the last 24 h. DC2.4 cells were co-cultured with 40 µg/ml of silica for 24 h for RT-PCR and ELISA such as in the case of exposure after differentiation. As shown in Fig. 3A~C, TNF-α expression in DC2.4 and BMDC was increased by all silica nanoparticles. However, they showed no effects on the mRNA expression of other cytokines including IL-1β or TGF-β. Secretion of TNF-α was enhanced by the exposure to silica (Fig. 3D and E), and production of IL-12p70 was below detectable levels by exposure to silica (Fig. 3F and G). Moreover, repeated exposure to silica in low dose after media change induced higher TNF-α production in DC than one exposure to silica in high dose (Fig. 3E). From these results, we have demonstrated that silica nanoparticles are able to increase the production of pro-inflammatory cytokine such as TNF-α in dendritic cells, and as a result, they may induce inflammatory responses.
Effect of silica nanoparticles on vessel formation and immune cell infiltration in matrigel plugs transplanted into mice in vivo
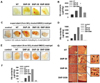
We also investigated whether silica nanoparticles induce inflammatory response in vivo. To observe vessel formation and immune cell infiltration by nanoparticle-induced inflammation, we used a growth factor-reduced matrigel. It is in liquid state at low temperature but becomes a gel at room temperature to form a genuine reconstituted basement membrane (23). The major components of this material are laminin, entactin, TGF-β, etc. With these characteristics, it has been used to support in vivo angiogenesis and recruitment of lymphocytes (23-26). First of all, we evaluated the effect of silica nanoparticles by itself on angiogenesis and lymphocyte infiltration in matrigel plugs transplanted into mice. As shown in Fig. 4A, silica nanoparticles induced vessel formation into the matrigel. In order to examine the recruitment of lymphocytes into matrigel, gel-formed matrigel was exchanged to liquid at 4℃ with Dulbecco's Phosphate Buffered Saline (DPBS) and subcutaneously injected into the abdominal region of mice. We could find some cells infiltrated into the matrigel, but cell population was not detectable because of its rare number. Lymphocyte infiltration was indirectly analyzed via the measurement of hemoglobin (Hb) concentration in matrigel. As a result, Hb concentration in matrigel was increased by a treatment with ultrafine silica particles, and silica with 1~5 µm size also induced an increased Hb concentration in matrigel compared to the non-treated controls. However, Hb concentration was the highest in the matrigel treated with silica particles, SNP-20 and SNP-50.
Next, we examined the effect of supernatant of silica nanoparticle-treated BMDC on vessel formation and Hb concentration in matrigel plugs when they are transplanted into mice in vivo. Supernatants were collected from BMDC exposed to silica nanoparticles which were added after differentiation or during differentiation. Those supernatants were concentrated 10-fold by centrifugation. Vessel formation and Hb content in matrigel plugs were increased by supernatant from BMDC co-cultured with silica nanoparticles in all cases (Fig. 4C~F). As shown in previous results, silica with a size of 1~5 µm had a relatively weak effect compared to ultrafine silica particles. These results indicate that silica nanoparticles increase the production of various inflammatory factors including TNF-α in dendritic cells and result in inflammatory responses in vivo.
We further evaluated whether vessel formation and immune cell infiltration in vivo can be found immediately by introducing DC2.4 cells exposed to silica. DC2.4-matrigel plugs were changed into highly dense tissues, and thick and long vessels were easily found in the matrix with naked eyes (Fig. 4G). As determined by H&E staining, vessel formation and lymphocyte infiltration were increased much more by silica nanoparticles. In particular, recruitment of red blood cells and formation of capillary vessel in DC2.4-matrigel plug were promoted by ultrafine silicas with a size of 10~20 nm and 50 nm. Therefore, data suggest that silica nanoparticles induce inflammatory responses mediated by dendritic cells, and inflammatory mediators from dendritic cells exposed to silica nanoparticles generate vessel formation and lymphocyte infiltration in vivo.
Activation of MAPK and NF-κB in dendritic cells by silica nanoparticles
MAPK and NF-κB are activated by several inflammatory stimuli, and they induce inflammatory gene expression. Thus, we determined whether silica nanoparticles lead to the activation of MAPK and NF-κB in DC2.4 cells. They were co-cultured with 40 µg/ml silica nanoparticles for 6 h. As shown in Fig. 5, silica nanoparticles enhanced the phosphorylation of ERK1/2, but did not activate SAPK/JNK. Especially, phosphorylation of p38 was highly increased by 10~20 nm and 50 nm ultrafine silica nanoparticles but was hardly induced by 1~5 µm silica particles. The production of IκB-α was also inhibited by ultrafine silica nanoparticles. Therefore, the data suggest that inflammatory responses by silica nanoparticles in dendritic cells are induced via MAPK and NF-κB activation.
DISCUSSION
In this study, we evaluated the effect of ultrafine silica nanoparticles on mouse bone marrow-derived dendritic cells (BMDC) and murine dendritic cell line, DC2.4. Silica nanoparticles are ranked as one of the top five commonly used nanomaterials in the nanotechnology consumer products by the Woodrow Wilson International Center for Scholars (27). It has been known that silica nanoparticles are able to enter the cells and be localized in the cytoplasm (28). Because dendritic cells play a role as gatekeeper in innate and adaptive immunity, it is important to study the effect of silica nanoparticles in dendritic cells.
10~50 nm sized ultrafine silicas decreased cell viability and proliferation, and induced apoptosis in DC2.4 cells. However, 1~5 µm sized silica particles had little toxic effect compared with ultrafine silica nanoparticles (Fig. 1). It has been reported that smaller particles have a higher level of cytotoxicity than larger particles. In human endothelial cells (EAHY926, a hybrid of human umbilical vein endothelial cells) and murine macrophage cells (RAW 264.7), smaller silicas showed much more inhibition of cell viability than larger silicas (29,30). However, study of Vallhov et al. has shown that a larger particle has more elevated cytotoxicity than a smaller particle in human dendritic cells (5). In contrast, Lin et al. and Cha and Myung reported that silica particles do not have a significant size-dependent difference of cell cytotoxicity in human lung cancer cells (A549), liver cells (Huh-7), brain cells (A-172), stomach cells (MKN-1), and kidney cells (HEK293) (31,32). Herein, our data also suggest that nanoparticles with 10~50 nm size do not show a significant size-dependent difference of cell cytotoxicity, although silica nanoparticles with 50 nm size showed more decreased cell viability than those with 20 nm size (Fig. 1). Therefore, size-dependent cytotoxicity of silica is yet to be confirmed.
Vallhov et al. investigated whether silica particles have immune modulatory effects on human monocyte-derived dendritic cells (MDDC) (5). They also indicated that CD86+ cells increased, but CD40+ cells and CD80+ cells decreased in immature MDDCs 24 to 48 h after co-culturing with silica particles. In our study, whereas CD11c+ cells were reduced, expression of CD86 and MHC class II was increased by exposure to silicas after differentiation of DCs (Fig. 2A). Moreover, it was additionally found that repeated exposures to silica nanoparticles inhibit the expression of surface molecules including CD11c, CD54, CD80, CD86 and MHC class II on mouse BMDC (Fig. 2B).
A number of reports have shown that inorganic nanoparticles induce inflammatory mediators and inflammatory responses in vitro. Inorganic layered metal hydroxide (LMH) and silica enhanced the release of IL-8 from human lung epithelial cells (L-132) and A549 carcinoma cells (33). Silver nanoparticles increased the production of TNF-α, MIP-2, and IL-1β in rat alveolar macrophages, too (34). In human bronchial epithelial cell line, BEAS-2B, titanium dioxide elevated not only the expressions of inflammation-related genes such as IL-1, IL-6, IL-8, TNF-α, and C-X-C motif ligand 2 (CXCL2), but also various oxidative stress-related genes including heme oxygenase-1, thioredoxin reductase, glutathione-S-transferase, catalase, and hypoxia inducible gene (35). In contrast to these results, single-walled carbon nanotube (SWCNT) inhibited the production of IL-6, IL-8, and MCP-1 in A-549 cells (36). In the present study, silica nanoparticles, similar to many previous works, induced a specific production of inflammatory cytokine, TNF-α, in dendritic cells (Fig. 3). Further, ultrafine silica nanoparticles led to activation of p38 and NF-κB in DC2.4 cells (Fig. 5).
Given that silicas are able to produce inflammatory cytokines in DCs and inflammation is involved in angiogenesis, we investigated the effect of silicas on vessel formation in vivo. Silicas induced vessel formation into the matrigel by itself (Fig. 4A and B). The supernatant from DCs exposed to silicas also triggered increased vessel formation and DCs exposed to silicas caused recruitment of red blood cells and formation of capillary vessel (Fig. 4C~G). However, silica with size of 1~5 µm had a relatively weak effect compared to ultrafine silicas (Fig. 4). Although we failed to confirm what kind of cells was infiltrated into matrigel, it was shown that angiogenesis was triggered by silica nanoparticles as well as the infiltrated cells into matrigel. In our previous report, silver nanoparticles (AgNPs) also induced angiogenic effect through activation of VEGFR pathway in vivo (26). Especially, AgNPs increased peritumoral vascularization in B16F10 melanoma model. Thus, it is required to consider a therapeutic possibility of nanoparticles including silicas and AgNPs in several diseases.
In conclusion, our findings suggest that silica nanoparticles, especially silicas with nanoscale size, have cytotoxic effects on dendritic cells and immune modulation effects in vitro and in vivo. Moreover, this study provides important information about the safety and immune regulation of ultrafine silica nanoparticles that are commonly used nanomaterials in the nanotechnology consumer products.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download