Abstract
Background
In this study, we have investigated the effect of Korean red ginseng (KRG) extracts on the production of TNF-α and IL-8 in human keratinocytes. Also, to examine the antioxidative effect of red ginseng extracts, free radical scavenging activity and superoxide dismutase (SOD) activity in human dermal fibroblasts was measured.
Methods
To investigate the effect of KRG in atopic dermatitis, we measured the level of TNF-α and IL-8 secretion in LPS-stimulated human keratinocytes after the treatment of KRG extracts using enzyme-linked immunosorbent assay. Anti-oxidative activity was investigated by measuring 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and SOD activity.
Results
The stimulation of human keratinocytes with KRG extracts shifted the LPS-induced cytokine secretion toward a more immunosuppressive response. KRG dose-dependently decreased TNF-α and IL-8 production in HaCaT cells and a significant inhibition of TNF-α was shown when cells were treated with 500 and 1,000 µg/ml of KRG extracts. Additionally, KRG extracts showed DPPH radical scavenging and SOD activity in a dose-dependent manner. Particularly, SOD activities of concentrations higher than 60 µg/ml of KRG extracts were significantly different in human dermal fibroblast cells.
All parts of the atopic disease have shown an increase in the last few decades (1). The first clinical manifestation of atopy is generally considered atopic dermatitis and the start of the atopic march. The atopic march is generally characterized by the progression of atopic dermatitis to asthma and allergic rhinitis during the first several years of life. The putative mechanism is the skin, which acts as the site of primary sensitization through possible defects in the epidermal barrier with later sensitization in the airways (2,3). Atopic dermatitis correlates with the expression of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-8, and IL-1 from keratinocytes, mast cells, and denderitic cells. Acute AD is associated with the production of T helper 2 type (Th2) cytokines but during chronic phase of AD, there is a switch to Th1-like cells which primarily produce TNF-α and IFN-γ (4).
Keratinocytes comprise 90% of the cells found in the epidermis and are capable of eliciting an immune response (5). Cytokines produced by these epithelial cells maintain normal homeostatic mechanisms in these structures. Skin cytokines can have pro-inflammatory as well as anti-inflammatory function, and dysfunction in the cytokine balances can contribute to inflammatory diseases in these organ systems (6). Keratinocytes synthesize and secrete a wide variety of cytokines including tumor TNF-α and IL-8. TNF-α is an important mediator of shock and cachexia (7). TNF-α induces fever, hypotension, prostaglandin E2 (PGE2), and local tissue necrosis (8). It also has a synergistic effect on PGE2-induced aggregation of neutrophils and synthesis of thromboxanes (6). In addition, IL-8 is a potent neutrophil chemoattractant and activating factor produced by a variety of cells including keratinocytes and its production is augmented by the primary cytokine TNF-α (9). Intradermally injected IL-8 induces neutrophil accumulation in dermis surrounding the dermal blood vessels (10). Overexpression of IL-8 is also thought to mediate T-cell chemoattraction in cutaneous T-cell lymphoma (11) and to contribute to neutrophil accumulation in the epidermis in psoriatic skin (12).
Moreover, antioxidants may be beneficial in conditions of oxidative stress, defined as when the oxidative/reductive balance of the body is tipped in favor of the former (13). Reactive oxygen species (ROS) released from inflammatory cells constitutes one of the critical causative factors in inflammatory skin diseases such as atopic dermatitis (14). Several types of inflammatory cells such as neutrophils and macrophages play an important role in acute inflammatory processes. However, in chronic and overaggressive inflammatory conditions, ROS may also be released into the extracellular compartment, and may cause local propagation of the inflammatory reaction and tissue damage (15,16). Therefore, the inhibition of ROS production or scavenging the released ROS may be important in preventing excess tissue damage in atopic dermatitis (14).
To treat atopic dermatitis, anti-inflammatory agents are often applied. Topical corticosteroids as anti-inflammatory agents rapidly reduce the severity of this disease, but allow early relapse of the dermatitis after discontinuing treatment. In addition, chronic use of stronger steroids may lead to a rebound phenomenon, steroid dermatitis, and rosacea-like lesions (17). Traditional herbs is an alternative therapy that can be used in the treatment of dermatologic disorders (18,19). Previous studies have shown that herbal therapy with oriental medicine may be beneficial for the treatment of patients with atopic dermatitis (20). Korean red ginseng (KRG, the steamed root of panax ginseng C. A. Meyer) is a well known traditional medicinal remedy used in Asian countries as well as in the United States and Europe. Active constituents with curable features found in most of ginseng species include ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids. Recent studies reported that major active ingredients such as ginsenosides promote anti-allergic effects and anti-inflammatory effects (21,22). However, few studies have investigated on their possibility as potential anti-atopic agents.
In this study, we have investigated the effect of KRG extracts on the production of TNF-α and IL-8 in human keratinocytes. In addition, to examine the antioxidative effect of red ginseng extracts, free radical scavenging activity and superoxide dismutase (SOD) activity in human dermal fibroblasts was measured.
Six-year old fresh ginseng was collected from Youngchun in Korea (October 2009). KRG extract was prepared by water extraction and manufactured by Coseed Biopharm (Korea). Briefly, red ginseng was made by steaming fresh ginseng at 95~100℃ for 2 h and drying at 55~60℃. One hundred grams of powdered red ginseng was added to 1 L of distilled water, and was extracted at 4℃ for 7 hr. The extraction procedure was repeated seven times. The extract was filtered using 400 mash and 0.45 µm filters. Sugar content and dry weight of the final extract was 1.7 brix and 0.44%, respectively.
Human keratinocytes (HaCaT) and human dermal fibroblasts (HDF-N) were maintained in Dulbecco's Modified Eagle Medium (DMEM) and were supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin at a temperature of 37℃ in a 5% CO2 humidified incubator (Sanyo, Japan). For cytoine production, cells were detached by vigorous pipetting. After centrifugation, the cells were incubated (5×105 cells/ml) with fresh medium in 24-well flat-bottomed tissue culture plates (Corning, NY, USA) at 37℃ in a 5% CO2 humidified incubator (Sanyo, Japan). Cells were supplemented in the absence or presence of KRG extracts and stimulants (1 µg/ml lipopolysachharide (LPS) for TNF-α and IL-8) for 48 h. Cultures were centrifuged at 300×g for 10 min to separate supernatant from cells.
Cell numbers and viability were assessed by trypan blue (Sigma, Poole, UK) dye exclusion. Twenty microliters of trypan blue solution was mixed with 20 µl of cell suspension in a microtube to obtain a final density of 0.3-2×106 cells/ml, and was loaded onto a hemocytometer. The cells that excluded the dye were counted in the standard manner within 1~5 min after mixing of the dye and cell suspension.
To determine the cytotoxic effect of KRG extracts, the viability of HaCaT and HDF-N cells were measured 48 h post-treatment. The cytotoxicity was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, Sigma, Poole, UK) assay colorimetric dye reduction method. Cells (1×105 cells/ml) were seeded in 96 well flat-bottomed tissue culture plates (Corning, NY, USA) in the absence or presence of KRG extracts for 24 h at 37℃ in a 5% CO2 humidified incubator (Sanyo, Japan). At the end of the incubation, 50 µl of filtered sterilized MTT stock solution was added and the plate was incubated for a further 4 h. Dimethylsulfoxide (DMSO, Duchefa Biochemie, Netherlands) was added and the absorbance was detected using an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, CA. USA) at 570 nm.
The levels of cytokine produced in the culture supernatants were determined by a commercially available ELISA kit obtained from Assay Designs, Inc (MI, USA). Briefly, supernatants were added to the 96-well plates coated with monoclonal antibody with specificity for human TNF-α or IL-8. Between subsequent steps in the assay, coated plates were washed 4 times with phosphate buffer saline (PBS) containing 0.05% (v/v) Tween-20. After exposure to medium, assay plates were sequentially exposed to biotin-conjugated anti-cytokine antibodies and detected by streptavidine-peroxidase anti-rabbit antibody. The plate was developed using 3,3',5,5' tetramethylbenzidine (TMB) and hydrogen peroxide solution and optical density readings at 450 nm was taken using an ELISA plate reader (Molecular Devices, CA, USA).
Various concentrations of KRG extracts were added to a methanolic solution of DPPH (0.1 mM) according to the method of Bois (23). After being stirred and left for 20 min at room temperature, the absorbance of DPPH solution was adjusted at 0.700±0.02 with 80% methanol at 517 nm. Fifty microliters of extracts or standard antioxidant solution was added to 2.95 ml of methanolic DPPH solution. The mixture was shaken vigorously and was left in the dark at 23℃ for 30 min. The amount of DPPH remaining was determined at 520 nm, and the radical scavenging activity was obtained from the following equation: Radical scavenging activity (%)={(ODcontrol-ODsample)/ODcontrol}×100.
SOD activity was assayed using the nitroblue tetrazolum (NBT) method of Beauchamp and Fridovich (24). Cells were homogenized in 0.05 M sodium carbonate buffer (pH 10.2). The assay mixture consisted of 0.05 M sodium carbonite buffer (pH 10.2) containing 3 mM xanthine, 0.75 mM NBT, 3 mM EDTA, 1.5 mg/ml BSA and 50 µl of homogenate. The reaction was initiated by adding 50 µl of xanthine oxidase (0.1 mg/ml) and incubated for 30 min at room temperature. The reaction was stopped by adding 6 mM of copper (II) chloride and centrifuged at 1,500 rpm for 10 min. The absorbance of formazan at 560 nm was then measured in the supernatant. The SOD activity was obtained from the following equation: SOD activity (%)={(ODcontrol-ODsample)/ODcontrol}×100.
All values expressed as mean±S.E.M. were obtained from at least five observations and were compared using one way ANOVA followed by Duncan's multiple range test. Differences were significant; probability values of <0.001, <0.01, or <0.05 were considered significant with 99.9%, 99% or 95% of confidence, respectively.
Prior to studying the cytotoxic effects of KRG extracts, we determined the optimal dose of LPS. Cells were treated with various concentrations of LPS and the optimal dose of LPS used (1 µg/ml) was determined from LPS titration curves (data not shown). Cells were treated for 48 h with KRG extracts at the indicated dose and the viability was measured using MTT assay. The control is shown in the bar graph. More than 80% of the cells survived at concentrations lower than 1,000 µg/ml and 1,000 µg/ml in HaCaT and HDF-N cells, respectively (Figs. 1, 2).
TNF-α and IL-8 are the major proinflammatory cytokines produced by keratinocytes, monocytes, and macrophages. As shown in Fig. 3, the stimulation of human keratinocytes with KRG extracts shifted the LPS-induced cytokine secretion toward a more immunosuppressive response. In the presence of KRG extracts (50~1,000 µg/ml), a decreased release of TNF-α was observed compared with LPS controls. A significant inhibition of TNF-α was shown when cells were treated with 500 and 1,000 µg/ml of KRG extracts (p<0.05). The negative control did not produce any cytokines. In addition, KRG extracts (50~1,000 µg/ml) dose-dependently decreased IL-8 production in HaCaT cells (Fig. 4). Overall, the levels of TNF-α and IL-8 released from LPS-stimulated HaCaT cells in the presence of KRG extracts showed a certain immunomodulatory effect. Since more than 80% of the cells survived at concentrations lower than 1,000 µg/ml in HaCaT cells, it seems unlikely that the inhibition of cytokine production was due to any cytotoxic effect of KRG extracts.
Decoloration of the stable free DPPH radical by antioxidant in samples was measured spectrophotometrically (Table I). In addition, we compared these results with those of α-tocopherol (Table II). According to the results, KRG showed that it inhibited DPPH formation by 50% at a concentration of 11 µg/ml (EC50). Samples showed DPPH radical scavenging activity in a dose-dependent manner.
In order to investigate whether the antioxidant activity of KRG extracts is mediated by an increase in antioxidant enzymes, we measured SOD activities in HDF-N cells treated with extracts of KRG which had previously shown relatively high DPPH radical scavenging activities (Table III). As a result, KRG samples dose-dependently increased the antioxidant enzyme activity of HDF-N cells. At a dose of 80 µg/ml, KRG extracts activated SOD level by 51.2%. SOD activities of concentrations higher than 60 µg/ml were significantly (p<0.05) increased (Table III). Since more than 80% of the cells survived at concentrations lower than 1,000 µg/ml in HDF-N cells, it seems unlikely that the induction of antioxidative activity was due to any cytotoxic effect of KRG extracts.
Atopic dermatitis is common inflammatory condition of the skin, often appears first in early infancy and may persist into adulthood. Although it is not life threatening, it affects the quality of life (25). For the management of atopic dermatitis, early treatment at the onset of symptoms and the prevention of recurrence are important. Use of topical or systemic steroid is essential for the successful management of atopic dermatitis; however, there are controversies regarding its long-term use (26,27). To date, several natural substance based anti-atopic agents have been topically tested as potential therapeutics for atopic dermatitis (28). Ginseng is known to possess various biological activities including anti-inflammatory and anti-tumor actions. The major components of raw ginseng are ginsenosides (29). The anti-allergic and anti-inflammatory effects of ginsenosides Rh1, anti-allergic effect of ginsenosides Rh2, and anti-allergic and anti-contact dermatitis activity of KRG saponin fraction and ginsenosides Rg3, Rf, and Rh2 have also been identified. The anti-allergic properties of KRG saponin fraction and ginsenosides have strongly suggested their possibility as potential anti-atopic agents (21,30). In this study, we have demonstrated that KRG extracts significantly suppressed LPS-induced elevation of TNF-α and IL-8 secretion in human keratinocytes (HaCaT).
Cultured human keratinocytes produce TNF-α following stimulation with lipopolysaccharide (LPS) (31), which can activate neutrophils, eosinophils, and macrophages (32). TNF-α induces fever, hypotension, leucopenia, local tissue necrosis, PGE2, and collagenase synthesis, fibroblast proliferation, and collagen synthesis (8). TNF-α and other proinflammatory cytokines produced at the initiation stage of atopic dermatitis induce the expression of a variety of chemokines and adhesion molecules which direct the recruitment, proliferation, and survival of leukocytes within the skin. KRG extracts significantly suppressed LPS-induced elevation of TNF-α production in human keratinocytes. Furthermore, Giustizieri et al. (33) reported that TNF-α is the most potent inducer of IL-8, therefore the production of this cytokine in human keratinocytes was also measured. Keratinocytes are the major cell population of the epidermis and can be activated to produce various chemokines, including IL-8/CXCL8 (34,35). Keratinocyte production of IL-8 has been implicated in the pathology of skin diseases (36,37). In this study, we confirmed keratinocyte-associated IL-8 production when cells were stimulated with LPS. High levels of IL-8 expression have already been implicated in multiple aspects of the pathogenesis of skin diseases, including T-cell and neutrophil chemotaxis and activation, as well as keratinocyte hyperproliferation (38). In vitro studies have shown that IL-8 was the only chemokine constitutively produced by keratinocytes, and TNF-α was the most potent inducer of IL-8 (33). Therefore, we can assume that the inhibition of TNF-α in LPS-stimulated keratinocytes has lead to the decrease of IL-8 production which may lead to the prevention of skin inflammation.
ROS produced by the activation of primed inflammatory cells have potentially deleterious effects on the biological system as they can damage proteins, lipids and nucleic acids (39-41). It was reported that the capacity for SOD induction in peripheral blood leucocytes in atopic dermatitis patients is lower than normal (42). Also, it has previously been shown that in extreme oxidative environments, H2O2 inactivates SOD and O2- inactivates the other scavenging enzymes such as catalase and glutathione peroxidase (43,44). Therefore, it is important to look into the SOD activity produced by KRG extracts to determine whether it can be considered a potential agent for atopic dermatitis. As shown in Table III, KRG extracts showed SOD activities in a dose-dependent manner. KRG extracts activated SOD level by 51.2% at a dose of 80 µg/ml and SOD activities of concentrations higher than 60 µg/ml were significantly (p<0.05) elevated. Since it was shown that environmentally generated ROS may induce oxidative protein damage in the stratum corneum, leading to the disruption of barrier function and exacerbation of atopic dermatitis (45), we investigated the scavenging activity of KRG to determine whether it beneficial to atopic dermatitis. In inflammatory conditions, activated macrophages and eosinophils produce ROS including O2-, H2O2, HOCl, and OH. These in turn encourage more cell migration into the area by stimulating cytokine release, adhesion molecule expression and complement activation (46,47). We tested the radical-scavenging effect of KRG extracts on ROS generated by the stable radical DPPH, and as result, KRG extracts showed a radical-scavenging activity in a concentration-dependent manner compared to the controls (Table I). EC50 was shown at 11 µg/ml of KRG, inhibiting the DPPH formation by 50%. Our results suggest that KRG extract can reduce the level of ROS released from inflammatory cells, which might contribute to their anti-inflammatory action.
In conclusion, our results demonstrates that KRG extracts suppress cytokines including TNF-α and IL-8 in LPS-stimulated human keratinocytes, and also has an antioxidative effect in human dermal fibroblasts. Based on our findings, KRG extracts may be considered a potential agent in the management of atopic dermatitis. Further studies are required to elucidate the mechanism of action of KRG extraction on atopic dermatitis.
Figures and Tables
 | Figure 1Cell viability of human keratinocyte cells (HaCaT) when treated with Korean red ginseng (KRG) extracts. HaCaT cells were treated with various concentrations (100, 200, 400, 800, 1,000, 2,000, 4,000, 6,000, 8,000, and 10,000 µg/ml) of KRG extract for 48 h and the cytotoxicity was measured by MTT assay colorimetric dye reduction method. |
 | Figure 2Cell viability of human dermal fibroblasts (HDF-N) when treated with Korean red ginseng (KRG) extracts. HDF-N cells were treated with various concentrations (100, 200, 400, 800, 1,000, 2,000, 4,000, 6,000, 8,000, and 10,000 µg/ml) of KRG extract for 48 h and the cytotoxicity was measured by MTT assay colorimetric dye reduction method. |
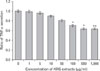 | Figure 3Inhibition of TNF-α secretion in HaCaT cells by Korean red ginseng (KRG) extracts. Cells were stimulated with 1 µg/ml LPS and was treated with various concentrations of KRG extracts for 48 h. Significant difference in comparison with control at *p<0.05 and **p<0.01. |
 | Figure 4Inhibition of IL-8 secretion in HaCaT cells by Korean red ginseng (KRG) extracts. Cells were stimulated with 1 µg/ml LPS and was treated with various concentrations of KRG extracts for 48 h. *Significant difference in comparison with control at p<0.05. |
ACKNOWLEDGEMENTS
This research was supported by a grant from Daegu Haany University Ky·lin Foundation.
References
1. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008. 121:947–954.

2. Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010. 105:99–106.

3. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003. 112:6 Suppl. S118–S127.

4. Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006. 118:178–189.

5. Pivarcsi A, Nagy I, Kemeny L. Innate immunity in the skin: how keratinocytes fight against pathogens. Curr Immunol Rev. 2005. 1:29–42.

6. Feliciani C, Gupta AK, Sauder DN. Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med. 1996. 7:300–318.

7. Beutler B, Cerami A. Oppenheim II, Shevach EM, editors. Cachectin (tumor necrosis factor): an endogenous mediator of shock and inflammatory response. Immunophysiology; the role of cells and cytokines in immunity and inflammation. 1990. New York: Oxford University Press;226–237.
8. Tracey KJ, Vlassara H, Cerami A. Cachectin/tumour necrosis factor. Lancet. 1989. 1(8647):1122–1126.
9. Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987. 84:9233–9237.

10. Leonard EJ, Yoshimura T, Tanaka S, Raffeld M. Neutrophil recruitment by intradermally injected neutrophil attractant/activation protein-1. J Invest Dermatol. 1991. 96:690–694.

11. Wismer JM, McKenzie RC, Sauder DN. Interleukin-8 immunoreactivity in epidermis of cutaneous T-cell lymphoma patients. Lymphokine Cytokine Res. 1994. 13:21–27.
12. Schröder JM, Christophers E. Identification of C5ades arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest Dermatol. 1986. 87:53–58.

13. Sies H. Sies H, editor. Oxidative stress: introductory remarks. Oxidative Stress. 1985. London: Academic Press;1–7.

14. Nakashima T, Sato E, Niwano Y, Kohno M, Muraoka W, Oda T. Inhibitory or scavenging action of ketoconazole and ciclopiroxolamine against reactive oxygen species released by primed inflammatory cells. Br J Dermatol. 2007. 156:720–727.

17. Inoue Y, Isobe M, Shiohara T, Hayashi H. Inhibitory activity of CX-659S, a novel diaminouracil derivative, against the rebound phenomenon following withdrawal of corticosteroid therapy for chronic contact hypersensitivity responses. Int Arch Allergy Immunol. 2003. 131:143–152.

18. Artik S, Ruzicka T. Complementary therapy for atopic eczema and other allergic skin diseases. Dermatol Ther. 2003. 16:150–163.

20. Gao XK, Fuseda K, Shibata T, Tanaka H, Inagaki N, Nagai H. Kampo Medicines for Mite Antigen-Induced Allergic Dermatitis in NC/Nga Mice. Evid Based Complement Alternat Med. 2005. 2:191–199.

21. Bae EA, Trinh HT, Yoon HK, Kim DH. Compound K, a metabolite of ginsenoside Rb1, inhibits passive cutaneous anaphylaxis reaction in mice. J Ginseng Res. 2009. 33:93–98.

22. Ro JY, Ahn YS, Kim KH. Inhibitory effect of ginsenoside on the mediator release in the guinea pig lung mast cells activated by specific antigen-antibody reactions. Int J Immunopharmacol. 1998. 20:625–641.

23. Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958. 181:1199–2004.

24. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971. 44:276–287.

25. Fivenson D, Arnold RJ, Kaniecki DJ, Cohen JL, Frech F, Finlay AY. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002. 8:333–342.

26. Lee JH, Cho SH. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011. 133:810–817.

27. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004. 113:651–657.

28. Kang JS, Yoon WK, Han MH, Lee H, Lee CW, Lee KH, Han SB, Lee K, Yang KH, Park SK, Kim HM. Inhibition of atopic dermatitis by topical application of silymarin in NC/Nga mice. Int Immunopharmacol. 2008. 8:1475–1480.

29. Shibata S, Fujita M, Itokawa H, Tanaka O, Ishii T. Studies on the constituents of japanese and chinese crude drugs. Xi. Panaxadiol, a Sapogenin of Ginseng Roots. Chem Pharm Bull (Tokyo). 1963. 11:759–761.

30. Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004. 133:113–120.

31. Köck A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, Luger TA. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990. 172:1609–1614.

32. Collins T, Lapierre LA, Fiers W, Strominger JL, Pober JS. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986. 83:446–450.

33. Giustizieri ML, Mascia F, Frezzolini A, De Pità O, Chinni LM, Giannetti A, Girolomoni G, Pastore S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001. 107:871–877.

34. Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, De Pità O, Puddu P, Girolomoni G. IL-4 enhances keratinocyte expression of CXCR3 agonistic chemokines. J Immunol. 2000. 165:1395–1402.

35. Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999. 162:494–502.
36. Gillitzer R, Berger R, Mielke V, Müller C, Wolff K, Stingl G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J Invest Dermatol. 1991. 97:73–79.

37. Gottlieb AB, Luster AD, Posnett DN, Carter DM. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988. 168:941–948.

38. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, Krutmann J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998. 19:359–361.

39. Miller DM, Buettner GR, Aust SD. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990. 8:95–108.

41. Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986. 247:1–11.

42. Niwa Y, Iizawa O. Abnormalities in serum lipids and leukocyte superoxide dismutase and associated cataract formation in patients with atopic dermatitis. Arch Dermatol. 1994. 130:1387–1392.

43. Hodgson EK, Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975. 14:5294–5299.

45. Niwa Y, Sumi H, Kawahira K, Terashima T, Nakamura T, Akamatsu H. Protein oxidative damage in the stratum corneum: Evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. Br J Dermatol. 2003. 149:248–254.

46. Cornelius LA, Li LJ, Sepp N, Lawley TJ, Caughman SW. The effect of ultraviolet B (UVB) and reactive oxygen intermediates (ROI) upon dermal microvascular endothelial cell (EC) adhesion molecule expression. J Invest Dermatol. 1992. 98:596.
47. Gougerot-Pocidalo M, Revillard J. Fuchs J, Packer L, editors. Oxidative stress, cytokines, and lymphocyte activation. Oxidative Stress in Dermatology. 1993. New York: Marcel Dekker;187–211.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download