Abstract
Background
CD4+Fop3+ regulatory T cells (Tregs) are needed to maintain peripheral tolerance, but their role in the development of autoimmune arthritis is still debated. The present study was undertaken to investigate the mechanism by which Tregs influence autoimmune arthritis, using a mouse model entitled K/BxN.
Methods
We generated Treg-deficient K/BxNsf mice by congenically crossing K/BxN mice with Foxp3 mutant scurfy mice. The arthritic symptoms of the mice were clinically and histopathologically examined. The proportions and activation of CD4+ T cells and/or dendritic cells were assessed in the spleens, draining lymph nodes and synovial tissue of these mice.
Results
K/BxNsf mice exhibited earlier onset and more aggressive progression of arthritis than their K/BxN littermates. In particular, bone destruction associated with the influx of numerous RANKL+ cells into synovia was very prominent. They also contained more memory phenotype CD4+ T cells, more Th1 and Th2 cells, and fewer Th17 cells than their control counterparts. Plasmacytoid dendritic cells expressing high levels of CD86 and CD40 were elevated in the K/BxNsf synovia.
CD4+Foxp3+ regulatory T cells (Tregs) make up 5~10% of peripheral CD4+ T cells and are essential for maintaining peripheral self-tolerance (1). The transcription factor Foxp3 is not only a unique marker for Tregs but also is required for the development and activity of the Tregs. The importance of Tregs in preventing autoimmunity has been proven in scurfy mice and in human patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) (2,3). Both the mice and human mutants lack functional Tregs due to loss-of-function mutations in the Foxp3 gene, and the absence of these cells is accompanied by severe autoimmune responses affecting many organs. In addition, quantitative and functional defects in Tregs are associated with autoimmune disorders, such as type 1 diabetes, multiple sclerosis and systemic lupus erythematosus (4-6).
Tregs elicit their regulatory effects on other immune cells by diverse mechanisms. They were originally identified by finding that they inhibited the proliferation of other T cells as well as cytokine production by the latter (7). They also regulate the maturation of dendritic cells (DCs), so preventing T cell priming (8). Their suppressive activity depends on cell-to-cell contact and/or the production of cytokines. Apart from secondary lymphoid tissues, Tregs are also found at sites of inflammation, such as arthritic synovial tissue and diabetic pancreases, although their roles such locations remain unclear (9,10).
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that is characterized by anarchic remodeling of joint architecture (11). Arthritogenic T cells and autoantibodies that migrate into the joints elicit inflammatory responses and lead to progressive destruction of cartilage and bone. Although the cause of RA is poorly understood, it is thought to be due to an adaptive immune response to autoantigen are accompanied by an inflammatory response.
Several groups have attempted to identify the role of Tregs in the pathogenesis of RA. A study using a collagen-induced arthritis model showed that depletion of Tregs increased susceptibility to the disease, whereas the other study indicated that Tregs were not involved in controlling proteoglycan-induced arthritis (12,13). The results in humans have been more diverse, since Tregs from patients with RA have been variously shown to be fully functional, partially defective, or fully defective (14-16). Therefore, the role of Tregs in RA is still a matter of debate.
K/BxN is a murine model of RA that has many of the clinical and histologic features of the human disease (17). In a previous study, we found that K/BxN mice contained normal number of Foxp3+ Tregs (18), and that these cells efficiently suppressed the proliferation of naïve CD4+ T cells, and cytokine production by effector CD4+ T cells, in vitro. Despite the existence of functionally intact Tregs, K/BxN mice develop autoimmune arthritis. This prompted us to investigate the role of Tregs in the pathogenesis of K/BxN arthritis. We generated Treg-deficient K/BxN mice (referred to as K/BxNsf mice hereafter) and examined diverse aspects of arthritis in these mice. We found that Treg deficiency in K/BxN mice resulted in earlier onset and more aggressive progression of arthritis, and that this coincided with increased activity of autoreactive T cells in secondary lymphoid organs and synovial tissue. Moreover, Treg deficiency increased the frequency and maturity of DCs in the inflamed synovia. Thus, our results suggest that although the activities of Tregs are not sufficient to block the development of arthritis in K/BxN mice, they reduce the severity of the autoimmune arthritis by lowering the activity of CD4+ T cells and DCs in secondary lymphoid organs and synovia.
KRN TCR transgenic mice on a C57BL/6 background (K/B) were donated by Dr. Diane Mathis (17), and maintained in our facility. A cross between K/B and NOD mice generated transgenic progeny (K/BxN) and non-transgenic littermates (BxN). Female C57BL/6 mice bearing a scurfy (sf) allele (Jackson Laboratory, Bar Harbor, ME, USA) were backcrossed to NOD for more than 6 generations to generate NODsf congenic mice. A cross between female NODsf mice and male K/B mice generated K/BxN and K/BxNsf mice. All mice were maintained in a specific pathogen-free barrier facility at Hanyang University. The study was approved by institutional Animal Care and Use Committee.
The mice were inspected blind and disease severity was evaluated using a scoring system as previously described with modifications (19): 0, normal; 1, slight erythema and mild swelling confined to the mid-foot (tarsals) or ankle joint; 2, erythema and mild swelling extending from the ankle to the mid-foot; 3, erythema and moderate swelling extending from the ankle to the metatarsal joints; 4, intensive erythema and severe swelling encompassing the ankle, foot, and digits; 5, severe ankylosis of the ankle. In this system, the maximal score per mouse is 20 and scores are expressed as the mean arthritic index on a given day. The thickness of both hind ankles was measured axially across the malleoli using a caliper (Mitutoyo, Kanagawa, Japan). Hind paws were removed postmortem, fixed, and decalcified in Rapid-Cal Immuno solution (BBC, Stanwood, WA, USA). They were then embedded in paraffin, sectioned, and stained with H&E.
Single-cell suspensions of spleen, lymph node (LN) cells and synovial cells were stained with monoclonal Abs (mAbs) as previously described (18). The following mAbs were purchased from BD Biosciences (San Jose, CA, USA): anti-B220 (RA3-6B2), anti-CD4 (RM4-5), anti-IL-17 (TC11-18H10.1), anti-CD11c (R2-40), anti-CD11b (TC11-18H10.1), anti-CD40 (TC11-18H10.1), anti-CD86 (TC11-18H10.1), anti-CD3 (TC11-18H10.1), anti-NK1.1 (TC11-18H10.1), and anti-γδ TCR (B56). The following mAbs were obtained from eBioscience (San Diego, CA, USA): anti-CD80 (16-10A1), anti-CD25 (7D4), anti-Foxp3 (FJK-16s), anti-IFN-γ (XMG1.2), and anti-IL-4 (BVD6-24G2). The mAbs conjugated with FITC, PE, PerCP, or allophycocyanin were used.
Hind paw tissues were fixed, embedded in paraffin, and sectioned at 7 µm in thickness. Endogenous peroxidase was inactivated by treatment with 3% H2O2 in absolute methanol. After blocking nonspecific binding with normal goat serum in PBS, the sections were incubated with rabbit Ab to receptor activator of nuclear factor kappa-B (RANK; Santa Cruz Biotechnology, CA, USA) or RANK ligand (RANKL; Santa Cruz Biotechnology) at appropriate dilutions. The sections were incubated with secondary Ab and exposed to avidin-biotin-peroxidase complexes and 3,3'-diaminobenzidine (Dako, Carpinteria, CA, USA). The sections were counterstained with hematoxylin, and the samples were photographed with a photomicroscope (Olympus, NY, USA).
Synovial tissues from inflamed ankle joints of K/BxN and K/BxNsf mice were minced using scissor and digested with 0.5 mg/ml of Liberase Blendzyme II (Roche Diagnostics, Indianapolis, IN, USA) in plain RPMI 1640 medium for 45 min at 37℃. Digested tissue was washed three times in sterile plain RPMI and filtered through a cell strainer to collect single cell suspension.
Mouse glucose-6-phosphate isomerase peptide (GPI282-294; LSIALHVGFDHFE) was produced by Peprotech (Daejeon, Korea) (18). Splenocytes (105/well) from K/BxN or K/BxNsf mice were cultured in the presence of irradiated BxN splenocytes (105/well) as APC and the indicated concentrations of GPI282-294. After pulsing with 3H-thymidine (0.5 µCi/well; Amersham Biosciences, Piscataway, NJ, USA) for the last 9 hours of a total of 72 hours of culture, 3H-thymidine incorporation was assayed. All assays were conducted in triplicate.
We found several differences between the arthritic symptoms of K/BxNsf mice, lacking Tregs, and their Treg-containing K/BxN littermates. The onset of arthritis occurred earlier and disease progression was accelerated in K/BxNsf mice (Fig. 1A). Ankle thickness, a measure of joint swelling, peaked 8 days earlier in K/BxNsf mice than in K/BxN mice. Despite the earlier decline of ankle thickness, joint deformity as indicated by arthritic indices and histological examination was much more severe in K/BxNsf mice than in the K/BxN mice up to 14 weeks of age (Fig. 1A and B). For example, the metatarsal bones were already invaded in the K/BxNsf mice at 4 weeks of age, while they were intact in K/BxN mice of the same age (Fig. 1B).
RANKL is a member of the TNF ligand superfamily, which is expressed by activated T cells and osteoblasts (20). RANKL binding to its unique receptor RANK activates the formation and function of osteoclasts, leading to bone resorption and loss. We performed immunohistochemical analyses to detect RANKL- and RANK-expressing cells in the hind paw joints of 6-week-old K/BxN and K/BxNsf mice. RANKL+ and RANK+ cells were much more abundant in the synovial tissues of the K/BxNsf mice than in those of the K/BxN mice (Fig. 1C). Thus, these results demonstrate that Tregs play a role in damping the severity of inflammation and bone destruction in the K/BxN arthritic model.
We examined whether Treg deficiency affected the number and/or activity of arthritogenic T cells. Total CD4+ T cell numbers were significantly higher in the spleens and joint-draining LNs (dLNs) of 4-8 week-old K/BxNsf mice than in their K/BxN littermates (Fig. 2A). These cells multiplied more than those from K/BxN mice even in the absence of exogenous GPI282-294 peptide, displaying enhanced self reactivity against endogenous GPI peptide (Fig. 2B).
We have shown previously that K/BxN mice contain numerous homeostatically proliferating CD4+ T cells retaining memory-like phenotypes, and that these cells are distinct from conventionally activated cells. Consistent with this, the large majority of CD4+ T cells populating the spleens and dLNs of K/BxN mice had memory (CD44hiCD62LloCD25-) phenotypes, while a few had undergone conventional activation (CD44hiCD62LloCD25+) or remained naïve (CD44loCD62Lhi) (Fig. 2C). The most prominent characteristic of the K/BxNsf mice was an increased frequency and absolute number of memory phenotype CD4+ cells in their spleens and dLNs (Fig. 2C and D). There were no comparable increase in conventionally activated CD4+ T cells in K/BxNsf mice (Fig. 2D).
To determine whether Treg deficiency affects the development of effector helper T (Th) cells, we assessed the profiles of IFNγ-, IL-4-, or IL-17-producing Th1, Th2 and Th17 cells, respectively, in the spleen and dLNs of BxN, K/BxN and K/BxNsf mice. The K/BxNsf mice contained significantly more Th1 and Th2 cells in their spleens and dLNs than K/BxN mice (Fig. 3A and C), while the proportion of Th17 cells was lower than in K/BxN mice, even though there was no difference in the absolute number of Th17 cells. Immunofluorescence staining confirmed the existence of effector T cells in the spleens of K/BxN and K/BxNsf mice, especially in the extrafollicular areas but not in the follicular areas (Data not shown).
Thus our results show that Treg deficiency results in dramatic expansion of memory phenotype CD4+ T cells, presumably homeostatically proliferating T cells, in response to endogenous GPI self antigen. Treg deficiency also results in population of T cell zone by Th1 and Th2 cells but not Th17 cells. The latters seem not to be involved in the germinal center reaction.
We examined whether Treg deficiency affects synovial cellularity in the K/BxN mice. To this end, we first tested whether Tregs are present in the synovial tissue of K/BxN mice. While Tregs were not seen in synovial tissue in the steady state, in the inflamed synovial tissue of K/BxN mice approximately 40% of the CD4+ T cells were Foxp3+ Tregs (Fig. 4A). Therefore, we suspected that Tregs play a substantial role in the pathology of the synovia.
To determine whether the absence of Tregs affects the homing of effector T cells to inflamed synovial, we examined the profiles of synovial T cells in K/BxNsf mice. As in the secondary lymphoid organs, K/BxNsf synovia contained more IFNγ+ Th1 cells and IL-4+ Th2 cells and fewer IL-17+ cells than K/BxN synovia (Fig. 4B). Since IL-17 can be produced by NK T cells and γδ T cells in addition to Th17 cells, we further characterized the phenotype of the IL-17-producing synovial cells. The majority of these cells in the arthritic joints expressed CD3 and γδ TCR, while few if any expressed CD4 or NK1.1 (Fig. 4C). Taken together, these results demonstrate that a deficiency of Tregs facilitates the influx of Th1 and Th2 cells into arthritic joints and does not affect the frequency of IL-17-expressing synovial γδ T cells.
Two major subsets of DCs, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), are abundant in the synovial fluid and perivascular regions of synovial tissues from patients with RA, suggesting that these DCs play an important role in inflammation. Hence, we tested whether Treg deficiency affected the DC subsets in the inflamed synovia. To this end, synovial cells extracted from arthritic joints of K/BxN and K/BxNsf mice were stained for CD11c, CD11b and B220. The proportion of CD11c+CD11b-B220+ pDCs was significantly higher and of CD11c+CD11b+B220- mDCs was slightly lower in K/BxNsf synovia than in K/BxN synovia (Fig. 5A). In addition, the expression levels of CD40 and CD86 were higher in the K/BxNsf pDCs than in those from K/BxN mice (Fig. 5B). The difference with regard to CD80 did not reach statistical significance. However, no upregulation of these molecules was seen in the mDCs of K/BxNsf mice (Data not shown). Thus, these results demonstrate that Treg deficiency leads to the accumulation of pDCs with higher costimulatory capacities in inflamed synovia.
Despite the existence of functionally intact Foxp3+ Tregs in K/BxN mice, these mice spontaneously develop arthritis. We were therefore prompted to consider the role of Tregs in these mice. We found that K/BxNsf mice lacking Tregs exhibited more serious symptoms of arthritis than their K/BxN littermates, including more extensive bone destruction, associated with increased proportion of RANKL+ cells in their synovial. They also contained more memory phenotype CD4+ T cells in their secondary lymphoid organs and synovia--- presumably proliferating homeostatically in response to autoantigens--- as well as more Th1 and Th2 cells. Importantly, pDCs with more mature phenotypes were more frequent in the synovia of the K/BxNsf mice than in those of the K/BxN mice. Thus, these results suggest that Tregs exert their effects on arthritogenic T cells and DCs, and so reduce the severity of disease.
We found that Treg deficiency resulted in general activation of T cells with the exception of Th17 cells. Unlike Th1 and Th2 cells, Th17 cells were more frequent in the K/BxN mice than the K/BxNsf mice. This result is intriguing in the light of the developmental and functional antagonism between Tregs and Th17 cells. We suspect that it may be due to the enrichment of Th1 and Th2 cells. Given that Th1 and Th2 cells can suppress the development of Th17 cells (21), such suppression may prevail over the direct effect of Treg deficiency. In addition, this result implies that Th17 cells do not play an important role in the exacerbation of arthritis in this model. Indeed, we observed that γδ T cells were more significant producers of IL-17 than Th17 cells.
We found above that Tregs, which are barely detected in synovia at steady state, became very numerous in inflamed synovia. This observation is in line with other reports showing enrichment of Tregs in inflamed tissues such as the synovial fluid of RA patients, pancreatic islets, colitis lesions, and the skin in graft-versus-host disease (22-25). This finding suggests that locally inflamed tissue provides a niche for Tregs to play a role in tissue-topical inflammatory responses. In this context, it would be of interest to further characterize the phenotypes and activities of synovial Tregs compared with those in secondary lymphoid organs.
An intriguing change in the Treg-deficient synovia was the increase in pDCs expressing elevated levels of CD40 and CD86. In agreement of this observation, others have detected the accumulation of pDCs in disease lesions in animal models and patients with autoimmune diseases, including type 1 diabetes, multiple sclerosis, Sjögren's syndrome and RA (26-29). Moreover, activated pDCs in local tissues preferentially secrete chemokines such as CXCL9, CXCL10, CCL3, CCL4 and CCL5, which can attract autoreactive T cells, so aggravating the disease (30-32). Similarly, we found that K/BxNsf synovia contained more Th1, Th2, and RANKL+ cells than K/BxN synovia. Therefore, we propose that synovial DCs are targets for the Treg activity that reduces synovial inflammation.
In conclusion, we have shown that Tregs reduce the severity of autoimmune arthritis by suppressing the activities of T cells and DCs in secondary lymphoid organs and synovial tissue.
Figures and Tables
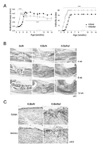 | Figure 1Accereration and exercerbation of arthritis in K/BxNsf mice. (A) Joint swelling in K/BxN and K/BxNsf mice was inspected blind. The ankle thickness of hind paws (left) and arthritic indices of all paws (right) are shown (n=6 mice/group). Data are means±SEM. *p<0.05, **p<0.01, and ***p<0.001 by Student's t-test. (B) Hind paws from BxN, K/BxN, and K/BxNsf mice were examined by histopathologic methods. Representative photographs of each group are shown. Original magnification, ×40. (C) Hind paw sections were stained with anti-RANK or RANKL Ab, followed by immunohistochemical processing. Original magnification, ×400. |
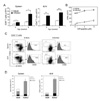 | Figure 2Treg deficiency enhances autoreactive activation of CD4+ T cells. (A) CD4+ T cell numbers in spleens and dLNs of K/BxN and K/BxNsf mice. *p<0.05, **p<0.01, and ***p<0.001 by Student's t-test. (B) Splenocytes purified from 4 week-old mice were cultured with irradiated splenocytes from BxN mice and GPI peptide at the indicated concentrations for 72 hr. 3H-thymidine was added during the last 9 h, followed by 3H-thymidine incorporation assays. Values represent mean±SD (C) Percentage of CD4+CD44loCD62Lhi and CD4+CD44hiCD62Llo cells in the spleens and dLNs of 5-10-week-old BxN, K/BxN, and K/BXNsf mice. Histograms represent the percentage of CD25+ cells among CD4+CD44hiCD62Llo cells. (D) Absolute numbers of naïve, memory and activated CD4+ T cells in spleens and dLNs of K/BxN and K/BxNsf mice (n=7). |
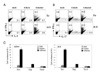 | Figure 3The profiles of Th1, Th2, and Th17 cells in the spleen and dLNs of K/BxNsf mice. (A and B) Splenocytes and dLN cells from 5 - 6 week-old BxN, K/BxN, and K/BxNsf mice were stimulated with PMA/ionomycin for 5 h and stained for CD4, IL-4, IFN-γ, and IL-17. FACS profiles gated on CD4+ cells are shown. A representative result of three independent experiments with the percentage of cells in each quadrant is shown. (C) Th1, Th2, and Th17 cell numbers in spleen and dLNs of K/BxN and K/BxNsf mice. |
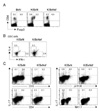 | Figure 4The profiles of synovial T cells from K/BxNsf mice. (A) Synovial cells extracted from ankle joints of 5 week-old BxN, K/BxN, and K/BxNsf mice were stained with mAbs to CD4 and Foxp3 and assayed by FACS. Representative FACS profiles gated on live lymphocytes are shown. (B) Synovial cells were stimulated with PMA/ionomycin for 5 h, followed by intracellular cytokine staining for CD4, IL-4, and IFN-γ. FACS profiles gated on CD4+ cells are shown. (C) Synovial cells were stimulated with PMA/ionomycin for 5 h and stained for IL-17, CD4, CD3, NK1.1, and γδ TCR. One representative result of three independent experiments with the percentages of cells in each gate is shown. |
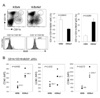 | Figure 5Enrichment of mature pDCs in the synovium of K/BxNsf mice. (A) Synovial cells extracted from ankle joints of 5 week-old BxN, K/BxN, and K/BxNsf mice were stained with mAbs to CD11b, CD11c, and B220. CD11c+CD11b+ cells (small circle) and CD11c+CD11b- cells (large circle) were gated for mDC and pDC, respectively. Histograms show the expression of B220 among each gate. Most CD11c+CD11b- pDC express B220 but most CD11c+CD11b+ mDC are B220 negative. The bar graphs on the right panel show the frequency of mDC or pDC on live lymphocyte gate. (B) The maturation status of CD11c+CD11b-B220+ pDCs was assessed by mean fluorescence intensity (MFI) of the co-stimulatory molecules CD40, CD80 and CD86. Bars and numbers indicate the mean of three animals. p-values in plots were calculated by Student's t-test. |
ACKNOWLEDGEMENTS
We thank Drs. Diane Mathis and Doo Hyun Chung for providing KRN mice. This work was supported by National Research Foundation grant funded by the Korean government (MEST; 2009-0081790).
References
1. Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.

2. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.

3. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001. 27:20–21.

4. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005. 54:92–99.

5. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004. 199:971–979.

6. Crispin JC, Martínez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003. 21:273–276.

7. Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005. 6:345–352.

8. Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004. 172:4676–4680.

9. Möttönen M, Heikkinen J, Mustonen L, Isomäki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005. 140:360–367.

10. Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005. 202:1387–1397.

12. Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003. 48:1452–1460.

13. Bardos T, Czipri M, Vermes C, Finnegan A, Mikecz K, Zhang J. CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis. Arthritis Res Ther. 2003. 5:R106–R113.
14. van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004. 50:2775–2785.

15. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.

16. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004. 200:277–285.

17. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996. 87:811–822.

18. Kang SM, Jang E, Paik DJ, Jang YJ, Youn J. CD4+CD25+ regulatory T cells selectively diminish systemic autoreactivity in arthritic K/BxN mice. Mol Cells. 2008. 25:64–69.
19. Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005. 17:325–333.

20. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999. 13:2412–2424.

21. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005. 6:1123–1132.

22. Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003. 33:215–223.

23. Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002. 16:183–191.
24. Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004. 173:3119–3130.

25. Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005. 201:1037–1044.

26. Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008. 105:12439–12444.
27. Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, Severa M, Parmentier M, Ristori G, Salvetti M, Aloisi F, Coccia EM. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008. 67:388–401.
28. Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, Labetoulle M, Ardizzone M, Sibilia J, Fournier C, Chiocchia G, Mariette X. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc Natl Acad Sci U S A. 2006. 103:2770–2775.

29. Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani GD, Minisola G, Tarantino U, Riccieri V, Valesini G, Coccia EM. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J Immunol. 2004. 173:2815–2824.

30. Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002. 169:6673–6676.





 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download