Abstract
Background
The active metabolite (1, 25-dihydroxycholecalciferol) of vitamin D (25-hydroxycholecalciferol) leads to activation of macrophages and deficiency of vitamin D seems to be involved in the risk of tuberculosis. The effects of vitamin D are exerted by interaction with the vitamin D receptor (VDR) and may be influenced by polymorphism in the VDR gene. In this study, variation in the VDR gene was investigated in Korean population with tuberculosis.
Methods
We typed three VDR polymorphisms of restriction endonuclease sites for TaqI, BsmI and FokI in 155 patients with tuberculosis and 105 healthy volunteers.
Results
The frequencies of FokI genotypes determined from TB patients were 29.13% for FF, 56.31% for Ff, and 14.56% for ff. We observed 1.4-fold increased prevalence of the Ff genotype in TB patients compared with normal healthy groups (p=0.0857). However, there was no significant association between the genotype groups, TB patient and normal control, for FokI polymorphism. There was also no significant association between VDR gene and tuberculosis in another polymorphism (BsmI and TaqI).
Most of the world's peoples are exposed and infected to a variety of mycobacterial species. Even if rarely pathogenic, mildly virulent mycobacteria such as Bacille Calmette-Guerin (BCG) and most mycobacteria may cause many different of clinical diseases. Mycobacterium tuberculosis and M. leprae, causing tuberculosis (TB) and leprosy, respectively, are more virulent. Remarkably, only a minority of individuals develops clinical disease, even though infected with virulent mycobacteria. Susceptibility to disease after infection by mycobacteria is influenced by environmental and/or host genetic factors. The interindividual variability of clinical outcome is thought to result, partially, from variability in the genes that control host defense (1,2).
Host genetic factors including major histocompatibility complex (MHC) polymorphisms influence both susceptibility to leprosy and TB (3-5). Non-MHC genes may also play an important role but remain undefined. Genetic variation studies of TB and leprosy have defined a role for HLA-DR (6,7) and variants of the NRAMP1 gene (8) in susceptibility to TB and leprosy.
Vitamin D has an immunoregulatory role mediated by binding to the vitamin D receptor (VDR) in monocyte, macrophages, and lymphocytes (9-11). Vitamin D status seems to be involved in the development of TB (12-15). VDR polymorphisms are occurred in several restriction enzyme sites. FokI and TaqI restriction sites are the best known polymorphisms of VDR gene (13,16). We, herein, investigated VDR genotype on susceptibility to TB and report an analysis of the polymorphisms in the FokI, BsmI, and TaqI restriction fragment length polymorphism (RFLP) of the VDR gene (Fig. 1) in TB and normal control samples collected from Korean population.
TB patients were recruited from St. Paul Hospital, Seoul, Korea from 2001 to 2002. M. tuberculosis infection was confirmed by AFB (Acid-Fast Bacilli) staining in their sputum and by culture. The control group comprised healthy, blood donors with no history of TB or other immune diseases.
The TB patients comprised 89 males and 66 females, and their ages ranged from 17 to 69 years. All patients were diagnosed with pulmonary TB and their mean duration of therapy was about 6.6 years. Healthy normal control subjects with no history of previous tuberculosis consisted of 67 males and 38 females, with age ranging from 21 to 52 years (Table I). Written consent was obtained from each participant.
The genomic DNA from the study subjects was isolated from PBMC. We prepared PBMCs from heparinized blood, isolated by centrifugation through Ficoll-Hypaque density gradient, then washed with DPBS (Sigma Chemical Co., St. Louis, MO. USA). 106 of PBMC were treated with PCR-K buffer [10× PCR buffer 1 ml, NP-40 40 µl, Tween-20 45 µl, protease K (20 mg ml-1) 30 µl, D.W. 8.8 ml] 1 ml, incubated at 58℃ for 1 hr and then incubated at 95℃ for 10 min to inactivate proteinase K. This product was used as a template DNA for PCR.
DNA was used in the PCR amplification of sequences containing previously described VDR restriction-fragment-length polymorphisms defined by the restriction endonucleases TaqI, FokI, and BsmI. The primer sequences used in this study were as follows: 5'-cag agc atg gac agg gag caa g-3' and 5'-ggt ggc ggc agc gga tgt acg t-3' for TaqI; 5'-agc tgg ccc tgg cac tga ctc tgc tct-3' and 5'-atg gaa aca cct tgc ttc ttc tcc ctc-3' for FokI; 5'-aac ttg cat gag gag gag cat gtc-3' and 5'-gga gag gag cct ctg tcc cat ttg-3' for BsmI.
The cycling profile involved denaturation at 94℃ for 15 sec, annealing at 65℃ (TaqI) and 55℃ (FokI and BsmI) for 30 sec, and extension at 72℃ for 30 sec for 35 cycles. Final extension was continued at 72℃ for 10 min. The amplification was carried out in a GeneAmp PCR system 9600 (Applied Biosystems, Branchburg, CT, USA).
PCR products were digested overnight with restriction endonuclease so the reaction could proceed to completion, in accordance with the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN, USA). Digested products were analyzed by electrophoresis in a 2% agarose gel and ethidium bromide staining.
The genotype frequencies of each of the SNPs were compared by the chi-square tests, Fisher's exact test and Cochran-Armitage Trend Test. Logistical regression analyses with three alternative models (additive, dominant and recessive) were used to calculate the odds ratios (OR) and 95% confidence intervals (CI) of each SNP. Data analyses were performed using the computer software SAS 9.1.3 (SAS Inc., Cary, NC, USA). All tests were two tailed and p<.05 was considered statistically significant.
The results of genotyping of VDR gene for TB patients and healthy control are summarized in Table 2 and 3. First, for the TaqI and BsmI polymorphism, the TB patient and normal control groups had similar distribution, and there was no significant difference in allele frequency distribution between patients and controls. In Table II, genotype frequency analysis of TaqI site in TB patients showed that the largest group consisted of TT homozygotes (89.93% of 149 genotypes), followed by the Tt heterozygous group (9.4%), while tt homozygotes were 0.67% (1 of 149 genotypes). In contrast, while bb homozygotes was the largest group (90.0% of 150 genotypes) in BsmI genotype, Bb heterozygote and bb homozygote are 8.67% and 1.33%, respectively.
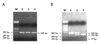
PCR products restricted with FokI were showed in Fig. 2. Three bands after treatment with enzyme were showed as FF homozygotes (266 bp), Ff heterozygotes (193 bp), and ff homozygotes (73 bp) according to the restriction pattern (Fig. 2). Table III showed the FokI genotypes determinants for TB patients and normal groups. The frequencies of the FokI genotypes were 29.13% for FF, 56.31% for Ff, and 14.56% for ff. We observed 1.4-fold increased prevalence of the Ff genotype in TB patients compared with normal healthy groups (p=0.0857). However, there was no significant association between the genotype groups, TB patient and normal control, for FokI polymorphism (Table III).
In human, mycobacterial pathogenicity varies from one mycobacterial species to another. M. tuberculosis and related species of TB complex are the agents of human TB, the leading infectious disease world-wide.
There is much variability among individuals in the response to mycobacterial infections, but it is not known why certain people develop disease when challenged with mycobacteria and others remain healthy. However, the intrinsic virulence of each mycobacterial species is not the sole pathogenic factor as the outcome of mycobacterial infection depends on the genetic backgrounds of the infected individual. The molecular basis of the genetic vulnerability underlying most mycobacterial diseases in human remains is, in large, unknown.
Recently genetic variation has been shown to be associated with single nucleotide polymorphisms (SNP) in the vitamin D receptor (VDR) gene in many populations of leprosy and TB (13,17,18). Epidemiological evidence suggests that there is a link between vitamin D deficiency and susceptibility to leprosy and TB.
Vitamin D metabolism leads to activation of macrophages and restricts the intracellular growth of mycobacteria. This effect of vitamin D may be influenced by polymorphisms at three sites (TaqI, BsmI and FokI) in the vitamin D receptor (VDR) gene (11). Recent studies also have implicated variation of the vitamin D receptor (VDR) gene in susceptibility to several diseases, including hepatitis and tuberculosis (17).
Therefore, we studied the association between VDR polymorphism and TB in Korean population with pulmonary TB and we found that there is no significant association in our analysis between TB patients and VDR polymorphisms. However, we found the differences of genotype frequencies of TaqI and FokI VDR between patients from Korean and the other countries including European, African, and Gujarati Asian previously reported (Table IV). For example, while the relative genotype frequencies of FokI polymorphism in Korean patients were FF 29.13, Ff 56.31, and ff 14.56%, the genotype frequencies in African were FF 46.34, Ff 43.45, and ff 8.21%. And the relative genotype frequencies of TaqI polymorphism in Korean were TT 89.93, Tt 9.4, and tt 0.67%, whereas the frequencies determined on Gambia (African) were TT 50.0, Tt 43.0, and tt 6.6%. However it is uncertain if there are racial differences involved in environmental or genetic factors.
Although several investigators reported and suggested that the VDR polymorphism may be of immunoregulatory importance for many disease processes, it is not clear that the polymorphism determine susceptibility to the development of clinical disease or susceptibility to infection.
Further studies will be required to investigate how VDR polymorphism may influence susceptibility to infectious disease or development of clinical disease.
Figures and Tables
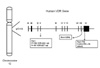 | Figure 1The human vitamin D recepter (VDR) chromosomal genes, containing a total 11 exons, are variably present in VDR transcripts. Three polymorphisms, including a FokI, BsmI, and TaqI site, are shown and discussed in the text. The figure is adapted from the schematic view in reference 4. |
 | Figure 2
FokI restriction patterns of the various genotypes. PCR products before (A) and after (B) treatment of enzyme were showed. B. Three restriction fragments were present at 266, 193, and 73 bp. The FF genotype (B-1) had only the 266 bp band, the ff genotype (B-3) had the two bands at 193 and 73 bp, and the Ff heterozygous genotype (B-2) had the three bands. |
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00112).
References
1. Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998. 101:2364–2369.

2. Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998. 280:1432–1435.

3. Wang LM, Kimura A, Satoh M, Mineshita S. HLA linked with leprosy in southern China: HLA-linked resistance alleles to leprosy. Int J Lepr Other Mycobact Dis. 1999. 67:403–408.
4. Kim SJ, Choi IH, Dahlberg S, Nisperos B, Kim JD, Hansen JA. HLA and leprosy in Koreans. Tissue Antigens. 1987. 29:146–153.

5. Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998. 338:640–644.

6. Rani R, Fernandez-Vina MA, Zaheer SA, Beena KR, Stastny P. Study of HLA class II alleles by PCR oligotyping in leprosy patients from north India. Tissue Antigens. 1993. 42:133–137.

7. Singh SP, Mehra NK, Dingley HB, Pande JN, Vaidya MC. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983. 148:676–681.

8. Abel L, Sánchez FO, Oberti J, Thuc NV, Hoa LV, Lap VD, Skamene E, Lagrange PH, Schurr E. Susceptibility to leprosy is linked to the human NRAMP1 gene. J Infect Dis. 1998. 177:133–145.

9. Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001. 15:2579–2585.
10. Rigby WF. The immunobiology of vitamin D. Immunol Today. 1988. 9:54–58.
11. Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998. 13:325–349.

12. Rook GA, Steele J, Fraher L, Barker S, Karmali R, ORiordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986. 57:159–163.
13. Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000. 355:618–621.

14. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006. 311:1770–1773.

15. Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007. 40:128–134.

16. Schauf V, Ryan S, Scollard D, Jonasson O, Brown A, Nelson K, Smith T, Vithayasai V. Leprosy associated with HLA-DR2 and DQw1 in the population of northern Thailand. Tissue Antigens. 1985. 26:243–247.

17. Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, Hill AV. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999. 179:721–724.

18. Roy S, Frodsham A, Saha B, Hazra SK, Mascie-Taylor CG, Hill AV. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999. 179:187–191.





 ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download