INTRODUCTION
Tuberculosis remains one of the most serious infectious diseases globally. In 2009, it had an estimated incidence of 9.4 million cases and 1.7 million people died of tuberculosis globally. Generally, one third of the world's population is thought to be latently infected (1). Upon infection, Mycobacterium tuberculosis invades and successfully replicate inside host macrophages. Although infected host cells can harbor viable mycobacteria, only ~10% of infected people develop tuberculosis (2). Therefore, the interaction between bacterial pathogenesis and the magnitude of the host immune response determines the outcome of the disease (3).
Earlier studies demonstrated the potential therapeutic roles of antimicrobial peptides in a variety of human diseases, including atopic dermatitis, cystic fibrosis and Crohn's disease (4). Recent studies have emphasized the roles of cathelicidin LL-37 in antimycobacterial immune defense, especially in human monocytes/macrophages (5,6). Vitamin D was found to be important in the regulation of LL-37 expression in monocytes, macrophages, and respiratory epithelial cells (6,7). Defensins have been widely studied as an antimicrobial peptide family present in airway fluid and reported to possess antimicrobial activities, including those against mycobacterial infection (8,9). Additionally, hepcidin, an antimicrobial peptide that regulates iron homeostasis, inhibits M. tuberculosis growth in vitro and inflicts structural damage on this notorious pathogen (10). Moreover, these antimicrobial peptide molecules influence a variety of physiological processes and also function as crucial signaling mediators in host defense and inflammation (11).
Despite these advances in research of the role of antimicrobial peptides in mycobacterial infection, the regulatory mechanisms of these antimicrobial peptides and their exact roles in inflammation during mycobacterial infection remain to be clarified. Thus, understanding the molecular mechanisms of expression of antimicrobial peptides and their role as immune modulators during the host innate response to mycobacteria will help in the design of new therapies against tuberculosis. Due to the increasing global incidence of multidrug-resistant tuberculosis, peptide-derived microbicides represent promising candidate therapeutics in the struggle against resistant mycobacteria.
GENERAL OVERVIEW OF ANTIMICROBIAL PEPTIDES/PROTEINS
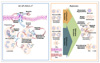
Antimicrobial defense peptides/proteins can be produced by activated macrophages and assist in elimination of ingested bacteria (4,5). Antimicrobial peptides such as cathelicidins and defensins play a crucial role in biological processes, including antimicrobial activities and immunomodulatory functions (summarized in Fig. 1). Cathelicidins are bipartite molecules consisting of an N-terminal cathelin domain and a C-terminal domain, which has antimicrobial activity (12,13). The N-terminal cathelin domain is known as a hallmark of the intracellular storage part of cathelicidins (12,13). Cathelicidins show constitutive and/or inducible expression in various cells and tissues (12,13). Their tissue/cell-specific expression is regulated by several stimuli including infection of microbes, inflammatory cytokines (13-15). Many studies reported that hCAP-18/LL-37 contributes to elimination of bacteria (14), systemic protection against microbial invasion (13), chemotaxis- attraction through secretion of several cytokines/chemokines (16), wound healing (16) and autophagy activation/maturation (17).
Defensins are antimicrobial/cytotoxic peptides which contain 29~35 amino acid residues, including 6 invariant cysteine residues (18). Defensins are expressed by various physiological/biological stimuli including TLRs or infection (18). The antimicrobial spectrum of defensins include not only gram positive microbes but also gram negative bacteria, including mycobacteria, Treponema pallidum, fungi, and some viruses (18-20). Defensins exert nonspecific antimicrobial/cytotoxic activity against mammalian target cells and microorganisms (18). In addition to their antimicrobial/cytotoxic properties, some defensins act as opsonins (18), contribute to selective chemo-attractants for monocytes (18), DCs and T cells (21), and promote to wound healing (8,22,23) and regulation of inflammation (24,25).
CATHELICIDIN hCAP-18/LL-37
The cathelicidin family, a key member of host defense peptide families, is derived from leukocytes and epithelial cells and has an important role in elimination of pathogenic microbes (13-15,26). Various inflammatory or infectious stimuli can induce cathelicidin LL-37, which then exhibits antimicrobial activity against a number of bacteria and fungi (12,14,22). hCAP-18/LL-37 is currently the only identified human cathelicidin, and profoundly affects multiple biological and pathological conditions (14,26). Cathelicidins contain a conserved N-terminal cathelin domain and a variable C-terminal cationic antimicrobial domain that becomes active and has antimicrobial activity. The mature peptide LL-37 comprises the C-terminal portion and is expressed in various cell and tissue types, including neutrophils, monocytes, keratinocytes, lymphocytes, and epithelial cells of the skin, testis, and the gastrointestinal and respiratory tracts (12,15).
Accumulating evidence supports an early defensive role for LL-37 at various sites. During mycobacterial infection, the highest expression of LL-37 was observed in alveolar macrophages (27). Other studies showed the importance of neutrophils in host defense against mycobacteria. When the overall immunity of blood cells to mycobacterial infection was evaluated in tuberculosis contactors, neutrophil counts were associated with a high risk of tuberculosis infection and restriction of mycobacterial growth (28). Additionally, the neutrophil peptides, cathelicidin LL-37 and lipocalin2 (Lcn2, also known as neutrophil gelatinase-associated lipocalin [NGAL]) contributed to inhibition of mycobacterial growth and immune defense against tuberculosis (28). Earlier studies showed that the synthetic peptide LL-37 had broad antimicrobial activity in airway epithelial cells of the lung (29) and in bronchoalveolar lavage fluids (30). Recently, we reported that M. ulcerans infection significantly induces antimicrobial peptide LL-37 in human primary keratinocytes via TLR2- and Dectin-1-dependent pathways (31). These reports emphasize a role for LL-37 in the early innate response to mycobacteria.
In mycobacterial infection of human mononuclear cells, LL-37 is induced in a vitamin D-dependent manner, and plays an important role in inhibition of intracellular mycobacteria through NADPH oxidase 2-dependent mechanisms (6,32). Similarly, M. bovis bacillus Calmette-Guérin (BCG)-induced up-regulation of the antimicrobial peptide cathelicidin LL-37 in human epithelial cells was dependent on NADPH/ROS signaling pathways (33). Of note, vitamin D is crucial for the regulation of LL-37 induction, which can be expressed in human monocytes and respiratory epithelial cells through conversion of vitamin D into its active metabolites (6,7).
Recently, emerging roles of autophagy in the regulation of innate immune functions have been reported (34). Importantly, the autophagy pathway has been known to be a key defensive mechanism to eliminate M. tuberculosis through phagosomal maturation (35). Our recent data have shown that vitamin D3 actively induces autophagy in human monocytes and inhibits intracellular mycobacterial growth through increased autophagosomal maturation (17). In this study, we found that LL-37 plays an important role in the induction and maturation of autophagy pathways activated by vitamin D3 in human monocytes (17). LL-37 regulated the transcriptional expression of the autophagy-related genes beclin-1 and atg-5 via C/EBP-β and MAPK activation (17). In addition, both defensin-β4 and cathelicidin are induced by distinct pathways in human monocytes, but cooperate to activate the TLR2/1-mediated antimycobacterial activity (9). Furthermore, recent studies have shown that the mycobacterial lipoprotein LpqH actively induces autophagy through functional vitamin D receptor signaling and following induction of LL-37-dependent pathways (36).
Besides autophagy regulation, additional novel functions of LL-37 have been reported: regulation of chemoattraction, inhibition of apoptosis, wound healing, angiogenesis, and release of cytokines/chemokines (15,16). LL-37 can also function as an immune regulator, mediating M. tuberculosis-induced ROS release and production of pro-inflammatory cytokines and chemokines (32). In mice, the only cathelicidin CRAMP was reported and structurally similar, but shorter, than human LL-37 (12). Both human LL-37 and murine CRAMP has a similar pattern of tissue distribution and biological function (15). For example, both LL-37 and CRAMP have been shown to be chemotactic for various immune cells, including neutrophils, monocytes, macrophages, and T cells (37,38). Further, increasing evidence indicates that various cytokines and signals affect induction of cathelicidin expression (16). The Th1 cytokine IFN-γ up-regulates, whereas the Th2 cytokine IL-4 down-regulates, TLR2/1-mediated induction of cathelicidin (39). In this way, cell-mediated immune responses can cross-talk with innate immune pathways via cathelicidin and other AMPs (39). Collectively, these data suggest that cathelicidin LL-37 exerts not only direct antimicrobial activity but is also an important immune modulator of autophagy regulation during mycobacterial infection.
DEFENSINS
Human defensins constitute a large portion of the pulmonary innate host defense system. Earlier studies showed that defensins are present in high concentrations on respiratory epithelia and selectively target microbial structures (40). Additionally, defensins function as signaling molecules, which link the adaptive immune system to invader microorganisms (40). Similar to cathelicidins, precursors of defensins that contain a characteristic β-sheet-rich fold and a framework of six disulfide-linked cysteines, require proteolytic cleavage for antimicrobial activity (41). The small (3~5 kDa) human cationic defensins are a delineated family of effectors of host defense, inflammation, and cytotoxicity (18). Defensins are divided into three subfamilies: α-, β-, and θ-defensins (25). Six α-defensins, four human β-defensins (HBD1~4) (reviewed in Ref. 25), and additional β-defensin gene clusters have been identified by computational analysis (42).
High-throughput studies using microarray analyses of gene expression profiles of PBMCs from patients with tuberculosis and M. tuberculosis-infected healthy donors found that the effector molecules α-defensin 1, 3, and 4 are upregulated in patients with tuberculosis (43). Human defensins show synergy with antituberculous drugs, thus suggesting that they may be a promising adjunct to antituberculous chemotherapy (44). Moreover, a protective role for α-defensin against mycobacterial infection has been reported in human eosinophils (19). α-defensin is induced in eosinophils upon stimulation with M. bovis BCG and lipomannan and shows a synergistic effect with eosinophil cationic protein on mycobacterial growth inhibition (19).
The human β-defensins HBD-1 and HBD-2 are predominately expressed at epithelial sites, and less well defined than α-defensin family (23,25). Both HBD-1 and HBD-2 has bactericidal activity against both Gram-positive and Gram-negative acteria (23,25). At least six HBD-1 isoforms (range in length from 36 to 47 amino acids) have been identified in urine, whereas a single isoform of HBD-2 (41 amino acids in length) has been isolated from respiratory epithelial secretions and saliva (23). While HBD-1 is constitutively expressed, HBD-2 is upregulated during bacterial infection or in response to endogenous inflammatory cytokines (23,45,46), suggesting a role for HBD-2 in regulation of antimicrobial and inflammatory responses. During mycobacterial infection, HBD-2 can be transported into mycobacteria-containing macrophage phagosomes to exert mycobactericidal and mycobacteristatic activity (20). In airway epithelial cells, HBD-2 mRNA is induced by M. bovis BCG infection and is upregulated by TNF-α produced by M. bovis BCG-infected cells (46). HBD-2 expression is also triggered by bacterial LPS/TLR4 stimulation through a CD14-dependent mechanism and ultimately results in activation of NF-κB (24).
Recent studies have emphasized the role of HBD4 in the innate immune defense against mycobacteria (9,35). TLR2/1-mediated IL-1β is required for up-regulation of HBD4 expression, which has antimicrobial activity against intracellular mycobacterial infection (9). Moreover, intratracheal administration of l-isoleucine into mice infected with the antibiotic-sensitive strain H37Rv and a multidrug-resistant clinical isolate significantly up-regulated β-defensins 3 and 4 and inhibited bacillary loads (47). M. bovis BCG-mediated expression of HBD2 was found to be regulated by the protein kinase C (PKC), JNK and PI3K/Akt pathways in airway epithelial cells (46). Of interest, more highly virulent M. bovis strains exhibit lower murine defensin-β4 expression, and vice versa, during early infection (48). In experimental tuberculosis, initial expression of murine defensin-β3 and defensin-β4 in airway epithelial cells was correlated with temporary control of mycobacterial growth (49). Additionally, high and stable production of mouse β-defensins, mBD3 and mBD4 during latent infection is associated with long-term control of mycobacterial proliferation (49).
Similar to hCAP-18/LL-37, HBD-2 plays roles other than direct antimicrobial action. These include chemotactic roles for immature dendritic cells and memory T cells through a chemokine receptor CCR6-dependent mechanism. This mechanism promotes adaptive immune responses by recruiting immune cells to sites of microbial invasion (21). Unlike α- and β-defensins, θ-defensins are found in some non-human primates, but not in humans, gorillas, bonobos, or chimpanzees (50). Tang et al. first isolated a trisulfide-containing antimicrobial peptide, termed rhesus theta defensin 1 (RTD-1), from granules of neutrophils and monocytes of the rhesus macaque (51). Although θ-defensins have antimicrobial activity against diverse pathogens (51-53), especially viruses (54-56), there is, as yet, no evidence that θ-defensins are involved in defense against mycobacterial infection. The roles of antimicrobial peptides in mycobacterial infection are summarized in Table I. Future studies will reveal the multiple roles of various human defensins in the regulation of immune responses and host defense against mycobacterial infection.
HEPCIDIN
Hepcidin is a cationic amphipathic bactericidal peptide primarily produced in the liver, and acts as a homeostatic regulator of iron absorption, recycling, and mobilization. Its expression is markedly induced during infectious and inflammatory conditions (57,58). Hepcidin is synthesized by iron loading and cytokine IL-6, and decreased by anemia and hypoxia. The major mechanism of hepcidin function is thought to be related to regulation of transmembrane iron transport through binding with ferroportin, an iron exporter expressed in hepatocytes and macrophages (59,60). The resulting decrease in extracellular iron concentrations probably makes less available for invading microorganisms and tumor cells, thereby contributing to host defense and controlling chronic diseases (57,58,61). As a novel mediator of innate immunity, hepcidin and related therapeutics are promising candidates for the treatment of various diseases, such as hemochromatosis and anemia from chronic inflammation (57,58,61).
Hepcidin is expressed in macrophages after infection with the intracellular pathogens M. avium and M. tuberculosis. Stimulation of macrophages with mycobacteria and IFN-γ synergistically induced hepcidin mRNA and protein, which localized to the mycobacteria-containing phagosomes (10). Additionally, hepcidin possesses direct antimicrobial activity and causes damage to M. tuberculosis (10). Further investigation of the signaling mechanisms responsible for hepcidin mRNA expression showed that STAT1 and NF-κB activation and induction of C/EBPβ were involved in IFN-γ and M. tuberculosis-induced hepcidin expression by macrophages (62). These data strongly suggest that M. tuberculosis-induced hepcidin expression by activated effector cells may contribute to host defense against mycobacteria. However, future studies are needed to clarify the exact roles and mechanisms of hepcidin expression in innate immune cells during mycobacterial infection.
CONCLUDING REMARKS
The antimicrobial peptides can contribute to antimycobacterial innate immunity through direct (killing) and indirect (immune modulation) activities. During mycobacterial infection, the cathelicidin, defensin, and hepcidin peptide families have been reported to exhibit antimicrobial activities and immunomodulatory functions. These peptides are produced in different types of innate immune cells, such as macrophages, neutrophils, keratinoyctes and epithelial cells. As a bridge between the innate and adaptive immune responses, cathelicidin may contribute to host antibacterial defenses and dampen harmful inflammation. Furthermore, antimycobacterial immune defense is linked to cathelicidin expression and its role in mediating autophagy. IL-1β, a crucial cytokine in antimycobacterial defense, is required for defensin expression, which is critical for innate immunity to mycobacteria. The accumulating data will enable development of therapeutic options and innovative antibiotics derived from host antimicrobial peptides.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download