It is well known that the accumulation of low-grade chronic inflammation is common in individuals who live a sedentary lifestyle and this condition has been linked to the development of various diseases including diabetes and cardiovascular diseases (1). Increased expression of toll-like receptor 4 (TLR4), a central innate immune receptor, has been observed in humans suffering from chronic inflammatory diseases such as obesity and type II diabetes (2,3). TLR4 is responsible for recognition of pathogens and plays an important role in innate immune function and adaptive immunity (4,5). It is well known that regular exercise is an effective countermeasure against low-grade chronic inflammation (6). It has recently been shown that exercise and regular physical activity exert anti-inflammatory effects through downregulation of TLR4 in the immune cells (7-9).
Because skeletal muscle is the largest organ in the human body and considered to be an endocrine organ due to the production of growth hormones and cytokines in response to exercise stimuli, it is worth examining the modulating effect of exercise on TLR4 in skeletal muscle cells. However, the molecular mechanisms by which exercise modulates TLR4 signaling pathways are currently unknown. Exercise induces various adaptations in skeletal muscle cells in terms of metabolic and hormonal aspects. Among them, increased levels of insulin-like growth factor-I (IGF-I) are one of the most well known adaptations induced by various exercise stimuli (10). Therefore, we investigated IGF-I to determine if it would modulate TLR4 gene expression and its downstream signaling and subsequently exert an anti-inflammatory effect on skeletal muscle cells. To investigate the possible relationship between IGF-I and TLR4 expression, we examined the mRNA and protein level of TLR4 in the presence or absence of IGF-I treatment. C2C12 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Welgene, Korea) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin) (Welgene, Korea). For the experiments, C2C12 myoblasts were plated in six-well culture plates at a density of 5×105cells/well in growth medium (DMEM, 10% FBS). For IGF-I treatment, cells at 90% confluence in DMEM supplemented with 2% horse serum (Hyclone, Logan, UT) and antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin; Welgene, Korea) were treated with IGF-I for 24 hr. The protein expression levels were then examined by Western blot analysis using anti-TLR4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-α-tubulin was also used to normalize the amounts of loading proteins.
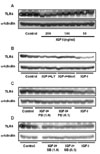
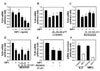
The results showed that IGF-I decreased TLR4 protein expression in a dose-dependent manner, indicating that IGF-I has a modulating effect on TLR4 protein expression in C2C12 skeletal muscle cells (Fig. 1A). The effects of IGF-I are believed to be mediated via signaling cascades including the PI3K/Akt and MAPK pathways (11). To determine if the PI3K/Akt pathway is involved in the IGF-I-mediated suppression of TLR4 protein expression, IGF-I-treated C2C12 myotubes were treated with specific PI3K/Akt inhibitors (LY294002 or Wortmannin). As shown in Fig. 1B, IGF-I-induced TLR4 protein suppression was significantly attenuated by LY294002 or Wortmannin. These data indicate that IGF-I mediates the suppression of TLR4 through PI3K/Akt signaling. However, as shown in Fig. 1C and D, when we used PD98059 (a specific ERK1/2 inhibitor) or SB203580 (a specific inhibitor of the p38 MAPK), we found that TLR4 expression levels were not significantly affected, indicating that p38 MAPK or ERK1/2 pathways are not involved in IGF-I-induced suppression of TLR4 protein expression. To determine if the modulating effect of IGF-I on TLR4 protein expression was associated with TLR4 gene expression, TLR4 mRNA levels were determined by real-time PCR. The results showed decreases in TLR4 mRNA in IGF-I-treated C2C12 cells of up to 73% with the maximum suppression occurring at an IGF-I concentration of 200 ng/ml (Fig. 2A). As shown in Fig. 2B and C, the suppression of TLR4 mRNA following IGF-I treatment in C2C12 cells was significantly attenuated by LY294002 (200 µM) or Wortmannin (100 nM and 200 nM). However, the suppressive effect of IGF-I on TLR4 mRNA expression was not significantly blocked by SB203580 (Fig. 2D) or PD98059 (Fig. 2E). Taken together, these results indicate that the suppression of TLR4 expression of both mRNA and protein in skeletal muscle cells is regulated by IGF-I, and that the negative-regulatory effect of IGF-I on TLR4 expression is regulated through activation of the PI3K/Akt pathways.
It is well known that TLR-mediated signaling activates NF-κB, which plays a critical role in regulation of the expression of pro-inflammatory genes such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (12). Given that IGF-I treatment suppresses TLR4 expression, we investigated whether IGF-I is also involved in the TLR4-mediated NF-κB-dependent pro-inflammatory gene expression. As basal cytokine gene expression is chronically elevated in individuals who live a sedentary lifestyle and have many chronic diseases associated with whole body chronic low-grade inflammation (1), we examined the basal expression level of IL-6 and TNF-α following IGF-I treatment. The results showed that IGF-I treatment greatly attenuated the endogenous expression of IL-6 and TNF-α, indicating that IGF-I exerts an anti-inflammatory effect on skeletal muscle cells by reducing the expression of pro-inflammatory cytokines under basal condition through down-regulation of TLR4 expression. Although the exact mechanism remains to be elucidated, we can speculate that cells having low TLR4 expression are less sensitive to endogenous inflammation-stimulating ligands such as heat shock proteins, which contributes low basal cytokine expression.
In the present study, we demonstrated that IGF-I treatment causes suppression of TLR4 expression in differentiating C2C12 skeletal muscle cells. Our data provide the first evidence that growth hormone is a potent modulator of TLR4 expression in skeletal muscle cells. It has been suggested that normal inflammatory responses are the natural host responses to an acute infection, whereas chronic inflammation is linked to many chronic diseases such as heart disease, some cancers, and type II diabetes (2,3,13). Regular exercise has anti-inflammatory effects and protects against diseases associated with chronic low-grade systemic inflammation. Skeletal muscle is now considered an endocrine organ and affects inflammation throughout the body via the production of pro-inflammatory cytokines (14). Therefore, it is possible that the IGF-I-induced suppression of TLR4 and cytokine expression in skeletal muscle cells observed in the present study may provide a mechanistic basis for the anti-inflammatory effect of exercise.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download