Abstract
Background
CM1 (centrocyte/-blast marker 1) was defined by a mAb against concanavalin A (Con A) activated PBMC. It is expressed in germinal center of human tonsil and on the surface of activated PBMC as well as cancer cells. Recently, increased productions of pro-inflammatory mediators were detected from activated PBMC by CM1 ligation.
Methods
However, there is a limitation to explain the exact role of CM1 on inflammation and its related mechanisms, since the identity of CM1 is still not clarified. In our previous study, we have already confirmed that soluble form of CM1 was produced by Raji. Therefore, we performed Q-TOF analysis after immunoprecipitation of concentrated Raji culture supernatant using anti-CM1 mAbs.
Results
As a result, we found that CM1 is identical to enolase-1(ENO1), a glycolytic enzyme, and we confirmed that results by silencing ENO1 using siRNA. It was also confirmed through competition assay between anti-CM1 and anti-ENO1 mAbs. Finally, we investigated the possible role of CM1 in inflammatory response and cancer. The ligation of CM1 on Raji cells with anti-CM1 mAbs induces the extensive production of prostaglandin E2(PGE2). In addition, the increased activity of matrix metalloproteinase (MMP)-2/9 was shown in NCI-N87, stomach cancer cell line by CM1 stimulation.
We have developed monoclonal antibodies against concanavalin A (Con A)-activated PBMC. One of monoclonal antibodies showed highly positive reaction in B cell area of germinal center of human tonsil by immunochemical staining. Since it is known that B cell area of germinal center is rich region of centrocyte/centorblast, we called the proteins recognized with monoclonal antibody made by ourselves as a centrocyte/-blast marker 1(CM1) (1). CM1 is not expressed on hematopoietic stem cells in bone marrow as well as immature thymocytes and PBMC. But after activation by PMA/ionomycin, more than 90% of peripheral B lymphocytes are became to CM1 positive, while 50~60% of peripheral T lymphocytes are became to CM1 positive (1). Its molecular size was originally detected at 70 kDa of membrane proteins, but it turned out that soluble form of CM1 at 40~45 kDa was also existed (2). With the regard of the function of CM1, it is thought that it is involved in the development and differentiation of B cells in secondary lymphoid tissues, especially germinal center in lymph nodes (1). In fact, the extensive apoptosis was found, when CM1 on CD38+IgD- germinal center B cells were ligated with anti-CM1 mAb (1).
In the case of Burkitt's lymphoma cell lines, Ramos and Raji, which have similar phenotypic profiles with normal B cells found in the germinal center, cell death were triggered by the cross-linking of CM1 on their surfaces through the destruction of mitochondria membrane potential and decrease of Bcl-2 expression (3). Increase of Fas expression was also found by the ligation of CM1 with anti-CM1 mAbs (4). Recently, expression of CM1 on Epstein-Barr Virus (EBV) transformed B lymphocytes were found (5). The same as the results with the ligation of CM1 on germinal center B cells and Burkitt's lymphoma cell lines, the extensive apoptosis was detected by the cross-linking of CM1 expressed on and EBV-transformed B lymphocytes with anti-CM1 mAbs, and it was closely related with the increase of reactive oxygen species (ROS) production (5). Therefore, it is thought that CM1 can be used as a novel centrocyte marker, during the developmental process of B cells in germinal centers through the regulation of apoptosis via the various kinds of apoptotic mechanism (4). However, all of experiments were done by anti-CM1 mAbs without determination of the identity of CM1.
Recently, there is a report regarding the role of CM1 on the pathogenesis of tumor (2). CM1 is highly expressed on the surfaces of stomach cancer, hepatoma and lung cancer. Moreover, its expression level is correlated with the malignancy of each kinds of tumor. Interestingly, we observed that soluble form of CM1 was increased in the serum of stomach cancer patients (2). Even though there are several evidences that might be closely related the progression of tumor, it was impossible to explain whether those results are novel findings by the novel molecules, because the identity of CM1 is not clearly determined so far.
Therefore, we first tried to clarify the identity of CM1 in the present study, and then we also investigated its possible roles on the regulation of the pathogenesis of inflammatory diseases and stomach cancer.
Human tumor cell lines, Raji (Burkitt's lymphoma) and NCI-N87 (stomach cancer) were used in this study and maintained in continuous log phase growth and cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100µg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). Normal human keratinocytes cell line, HaCaT, was used as a negative control for CM1 expression.
Balb/c mice were immunized with concanavalin A (Con A, Sigma, St Louis, MO, USA)-activated human PBMCs (1×107) at 2-week intervals for 2 months. Purified splenocytes (1×108) from spleen of immunized mice were fused with SP2/0-Ag14 mouse myeloma cells (1×107) using polyethylene glycol (PEG 4000; Sigma, St Louis, MO). The culture supernatant of antibody producing hybridoma cells were collected and screened through the immunohistochemical staining in serial frozen sections of human tonsil. The isotype of anti-CM1 mAb was determined by enzyme immunoassay using ScreenType (Boehringer Mannheim, Mannheim, Germany), according to manufacturer's protocol. Hybridoma cell of culture supernatant, which showed a strong positive reaction with tonsil section, was selected and injected into peritoneal cavity of Balb/c mice. And then anti-CM1 monoclonal antibody was purified from ascites by protein A column. MOPC-21 (Sigma, St Louis, MO, USA) was used as an isotype control.
Human Burkitt's lymphoma cell line, Raji was maintained in continuous log phase growth and cultured in serum-free culture medium (Protein-free hybridoma medium, Gibco, Grand Island, NY, USA) supplemented with 100 units/ml penicillin, and 100µg/ml streptomycin. Ten liters of culture supernatants were 10,000× concentrated by ultrafiltration using (Amicon, Millipore Corporation, Bedford, MA, USA). And then the proteins, which molecular weight is more than 100 kDa or less than 30 kDa, were removed via two separate steps of ultrafiltration (Microcon, Millipore Corporation, Bedford, MA, USA). Hundred microliter of concentrated culture supernatants were subjected to immnoprecipitation using twenty-five micrograms of anti-CM1 mAb and Dynabeads Protein G (Invitrogen Life Technologies, CA, USA). Immunoprecipitated proteins were electrophoresed on 10% SDS-polyacrylamide gel. The gel was subjected to silver staining kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's protocol and detected bands were analysed by Q-TOF Mass Spectrometry.
MS/MS of peptides generated by in-gel digestion was performed by nano-ESI on a Q-TOF mass spectrometer (AB Sciex Instruments, CA, USA). The source temperature was 80℃. A potential of 1 kV was applied to the precoated borosilicate nanoelectrospray needles (EconoTip™, New Objective, USA) in the ion source combined with a nitrogen back-pressure of 0~5 psi to produce a stable flow rate (10~30 nl/min). The cone voltage was 40 V. The quadrupole analyzer was used to select precursor ions for fragmentation in the hexapole collision cell. The collision gas was Ar at a pressure of 6~7×10-5 mbar and the collision energy was 20~30 V. Product ions were analyzed using an orthogonal TOF analyzer, fitted with a reflector, a micro-channel plate detector and a time-to-digital converter. The data were processed using an Analyst program.
To identify the protein, peptide masses from MS/MS were matched with the theoretical peptides of proteins in the NCBI database using MASCOT software. Also, all MS/MS spectra recorded on tryptic peptides derived from spot were searched against protein sequences from NCBInr and EST sequences from NCBInr and EST databases using the MASCOT search program (www.matrixscience.com).
Five micrograms of human enolase-1 recombinant protein (Abnoba corporation, Taipei, Taiwan) were added to SDS-loading buffer (0.5 M Tris-HCl (pH 6.8), 1 M 2-ME, 10% (w/v) SDS, 10% (v/v) glycerol, 0.05% (w/v) bromphenol blue) and boiled for 10 min. The boiled samples were then loaded into 10% polyacrylamide gels, electrophoresed and transferred to nitrocellulose membranes. Membranes were blocked overnight at 4℃ in PBS containing 0.1% (v/v) Tween 20 and 5% (w/v) non-fat milk proteins. Blocked membranes were then incubated with a mouse developed anti-CM1 mAbs for overnight at 4℃. And then washed three times (5 min each) with PBS containing 0.1% Tween 20, and incubated with a HRP-conjugated anti-mouse IgG secondary Ab (1:5,000; Cell signaling, Danvers, MA, USA) and detected with the ECL detection kit (Amersham, Piscataway, NJ, USA).
Raji cells were stimulated with anti-CM1 mAbs (1µg/ml) at room temperature with gentle rotation for 1 hr. Cells were transferred into 6-well plate and incubated for indicated times at 37℃ in a humidified incubator containing 5% CO2 and then cultured for another 24 hrs. The amount of PGE2 produced by CM1 stimulation was measured by ELISA (Cayman, Minneapolis, MN, USA), as manufacturer's protocols.
Human ENO1 siRNA and scrambled siRNA were purchased from SantaCruz (Palo alto, CA, USA). Human Burkitt's lymphoma cell line, Raji in exponential phase of growth were plated in T75 flask at 3×106 cells/flask, then transfected with 20, 40 nM of siRNA using effectene (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's protocol. The transfection efficiency by RT-PCR using specific primer for ENO1was determined at 72 hrs after transfection. Control cells were transfected with scrambled siRNA.
The expression of CM1 on several kinds of tumor cell lines was examined by flow cytometry analysis. Briefly, cells were washed twice with ice-cold PBS containing 0.05% BSA and 0.02% sodium azide. After two washes, cells were incubated with FITC-conjugated anti-CM1 mAbs or anti-ENO1 mAbs (SantaCruz, Palo Alto, CA, USA) for 30 min on ice. After two washes, cells were acquired on an FACS Calibur (BD Biosciences, San Jose, CA, USA).
To examine the activity of matrix gelatinases (MMP-2 and MMP-9), tumor cell were incubated in the presence or absence of anti-ENO1 mAb, and then lysates (10µg/lane) were loaded onto 10% gelatin gel of 0.75 mm thickness. After electrophoresis, the gel was washed with 2.5% Triton X-100 buffer containing 50 mM Tris (pH 7.4) and 5 mM CaCl2 for 1 hr at room temperature. Following a brief wash with distilled water, the gel was incubated with incubation buffer containing 50 mM Tris (pH 7.5), 200 mM NaCl, and 20 mM CaCl2 for 16 hrs at 37℃. The gel was stained with 0.2% Coomassie Blue for 1 hr, and then de-stained with a mixture of 30% methanol and 10% acetic acid for 2 hrs. Digested regions appeared as white bands on a blue background.
Two types of CM1 exist. One is 70 kDa of membranous form, and the other is 40~45 kDa of soluble form. Based on the reports that Burkitt's lymphoma cell lines, Raji and Ramos were expressed CM1 on the surface, we tried to identify CM1 through immunoprecipitation using membrane protein fraction from Raji. However, we could not obtain clear results, since CM1 was not dominantly expressed on the surface of Raji. Based on the reports that Raji and Ramos largely produced soluble CM1 (sCM1), CM1 was immunopreciptated using soluble protein fraction in this experiment. It is thought that sCM1 account for just a small part of soluble proteins, the mass culture of Raji was performed by using of protein-free culture media. five liters of culture supernatants were concentrated to hundred microliter of by ultrafiltration. Concentrated supernatants were subjected to immnoprecipitation, followed by electrophoresis on 10% SDS-polyacrylamide gel and silver staining. As shown in Fig. 1, we could find 3 bands, which assumed to react with anti-CM1 mAb, at near the region of 46 kDa. Those bands were extracted and subjected to Q-Tof analysis. One of the protein fractions is identical to enolase-1(ENO1), a glycolytic enzyme (Table I).
Even though ENO1 is expressed in the cytosol and known as a key enzyme in glycolysis, there are some reports that ENO1 is expressed on the surface of monocytic leukemia cell line, U937 and activated lymphocytes (6,7). Based on this report and our data, we confirmed whether CM1 is identical molecules with ENO1. First, we examined whether anti-CM1 mAb could detect recombinant ENO1. Anti-CM1 mAb successfully detected recombinant ENO1 (Fig. 2A). In addition, we did the competition assay between anti-CM1 mAb and commercially available anti-ENO1 mAbs. Raji cells were treated with 0.1µg of anti-ENO1 mAb prior to add 1µg of anti-CM1 mAbs. The binding activity of anti-CM1 mAbs was slightly decreased. However, its binding activity was more decreased by the addition of 0.2µg of anti-ENO1 mAb (Fig. 2B). It assumes that complete inhibition is not shown due to the different affinity of both of antibodies. However, these data are enough to determine that CM1 is an identical molecule with ENO1.
Next, we investigate the function of CM1 on the production of prostaglandin E2 (PGE2) from Raji cells, since we have already found the increase of pro-inflammatory cytokine production from Raji cells (data not shown). As shown in Fig. 3A, we found that extensive PGE2 production by the stimulation of CM1 on Raji. It suggests that CM1 plays a role in the pathogenesis of several kinds of inflammatory disease. ENO1's functions on the pathogenesis of inflammatory diseases and autoimmune diseases were already reported. To confirm whether CM1 is an identical molecule with ENO1 in terms of its functional activity, changing on the production of PGE2 by the transfection of ENO1 specific siRNA was examined. CM1-induced PGE2 production from the cells transfected with ENO1 specific siRNA was decreased in a dose-dependent manner (Fig. 3B). Therefore, we can conclude that CM1 is an identical molecule with ENO1 and CM1/ENO1 is involved in the production of one of inflammatory mediators, PGE2.
We have previously reported that CM1 is highly expressed on several kinds of tumor cells (2). However, ENO1 expression on tumor is not investigated. So, we examined the expression ENO1 on the surfaces of stomach cancer cell line, NCI-N87 with anti-ENO1 mAb and compared it when it stained with anti-CM1 mAb. There was a subtle difference on the detection of CM1/ENO1 by both of antibodies (Fig. 4A). As we described above, it might be due to the different affinity of both antibodies. It is well-known that matrix metalloprotease (MMP)-2/-9 is important factor for the metastasis of cancer through the remodeling of matrix around tumor. Therefore, we next examined the production of MMP-2/-9 from NCI-N87 after ligation of ENO1 with anti-ENO1 mAb. As shown in Fig. 4B, increased MMP-2/-9 activity was shown by the stimulation with anti-ENO1 mAb. It suggests that CM1/ENO1 also plays an important role on the metastatic process through the increase of MMP-2/-9 production of stomach cancer.
We have already investigated the function of CM1 on the development and differentiation of B cells thought the induction of apoptosis and its related mechanisms (1,4). However, it was very difficult to explain its importance on the pathogenesis, since the identity of CM1 was not clearly defined. Even though we tried to establish the identity of CM1 via the several kinds of molecular and cellular methods, we could not clearly defined due to the CM1 is inducible molecules under inflammatory and pathogenic condition. Moreover, it exists as a low abundance protein among membrane proteins.
In this experiment, we figured out the determination of the identity of CM1, based on its existence as a soluble form. We used Q-Tof analysis, because the most important factors for the identification of low-level of impurities and low abundance proteins is sensitivity and dynamic ranges for analysis (8,9). Q-Tof analysis is more confidence analysis for low abundance proteins and low molecular weight compounds. Finally, we succeeded to determine the identity of CM1 as α-enolase (ENO1).
ENO1 is one of the isotype of enolase family and others are β-enolase (ENO3) and γ-enolase (ENO2). Even though ENO1 is a key enzyme for glycolysis, there are several reports regarding the role of ENO1 in inflammatory responses and autoimmune diseases (10). Especially, the roles of ENO1 on the development and pathogenesis of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are well investigated (11-13). Expression pattern of ENO1 seems to show disease specific patterns. It suggests that ENO1 might be a target for the control of such kinds of diseases. In fact, we already observed that the symptoms and disease progression of RA have gotten better after regulation of ENO1 activity by anti-ENO1 mAbs or specific inhibitors for ENO1 function (our unpublished data). The roles of ENO1 mostly rely on its 1) immunogenic properties, 2) DNA-binding ability, and 3) Plasminogen receptor function. However, the specific role and function of ENO1 regarding the pathogenesis of autoimmune diseases remain to be clarified.
Apart from autoimmune diseases, the recent research regarding the roles of ENO1 are focused at cancers, infections and ischemia (10). Even though the data is not presented in this reports, we found the expression of ENO1 on several types of tumor cells including, stomach cancer, hepatoma, lung cancer, breast cancer, and prostate cancer. It seems that the expression of ENO1 is closely related with the malignancy of cancer cells, since higher expression on tumor shown high metastatic ability than cells showing non-metastatic ability was observed. As we shown in Fig. 4A, ENO1 expression is highly expressed on NCI-N87, stomach cancer cell line that shows prominent malignancy with high metastatic ability. In addition, ENO1 is also involved in the metastasis of cancer through the increase of MMP-2/-9 production. In fact, we found that the increase of CM1 (the name of ENO1 called before determination) production and expression in the serum and tissues of stomach cancer patients.
As we described shortly above, plasminogen is known as a ligand for membrane expressed ENO1 in vivo (14,15). It means that the inflammatory responses and MMP-2/-9 production can be actually triggered by plasminogen through the stimulation ENO1 on inflammatory mediator cells and cancer cells, respectively, even though we showed here by using of anti-ENO1 mAbs. However, we do not exclude the role of anti-ENO1 mAbs in vivo, especially on the development and pathogenesis of autoimmune diseases, since there are several reports regarding the increase of serum titer of anti-ENO1 mAbs in autoimmune disease patients. The serum levels of anti-ENO1 mAbs in cancer patients are now under investigation. Taken together, CM1/ENO1 might be a useful target for the therapy of autoimmune diseases and cancer through the development of anti-CM1(ENO1) antibody-based therapeutic reagents and appropriate inhibitors for function of membrane CM1/ENO1.
Figures and Tables
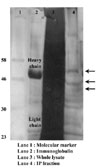 | Figure 1Immunoprecipitation of CM1 with anti-CM1 mAb. Ten liters of Raji culture supernatants were 10,000x concentrated by ultrafiltration, and then proteins, which molecular weight is more than 100 kDa or less than 30 kDa, were cuf-off by two separate steps of ultrafiltration. Hundred microliter of concentrated culture supernatants were subjected to immnoprecipitation by using twenty-five micrograms of anti-CM1 mAb and Dynabeads Protein G. Immunoprecipitated proteins were electrophoresed on 10% SDS-polyacrylamide gel and stained by silver staining kit according to manufacturer's protocol. Three bands (arrow) near at 46 kDa were selected and subject to further investigation by Q-Tof analysis. |
 | Figure 2Determination of CM1 as an identical molecule to ENO1. (A) Five micrograms of recombinant ENO1 protein was detected by anti-CM1 mAb. (B) Next, we investigated whether anti-CM1 mAb and anti-ENO1 mAb recognize spontaneously expressed ENO1 at the same time by the flow cytometric analysis. The binding capacity of anti-CM1 mAb (1µg) was gradually decreased by the addition of anti-ENO1 mAbs (0.1µg and 0.2µg). |
 | Figure 3Increase of PGE2 production by CM1 stimulation and its decreasing by ENO1 siRNA transfection. (A) Production of PGE2 was measure by ELISA as described in Materials and Methods. Briefly, cells were stimulated with anti-CM1 mAbs (1µg/ml) for 1 hr and further incubation for another 24 hrs. The amount of PGE2 produced by CM1 stimulation was measured by ELISA. (B) PGE2 production from ENO1-siRNA transfected Raji cells was measured, after stimulation with anti-CM1 mAbs. |
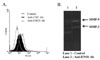 | Figure 4Expression of CM1/ENO1 on NCI-N87 and its role on the induction of MMP-2/-9 production. (A) The expression of CM1/ENO1 was examined by anti-CM1 mAb and anti-ENO1 mAb, respectably. Cells were incubated with FITC-conjugated anti-CM1 mAbs or anti-ENO1 mAbs and acquired on an FACS Calibur. (B) The increase of MMP-2/-9 production by the stimulation of anti-ENO1 mAb. To examine the activity of matrix gelatinases (MMP-2 and MMP-9), NCI-N87 was incubated with anti-ENO1 mAb. Digested regions are shown as white bands on a background. |
Table I
Result of Q-Tof analysis

Immnoprecipitated and separated proteins shown in Fig. 1 were eluted and subjected to be further analyzed by Q-Tof analysis as described in Materials and Methods.
ACKNOWLEDGEMENTS
This work supported by grant from Seoul National University College of Medicine (09' Basic and Clinical Cooperation Research Program: #800-20090024).
References
1. Hur DY, Kim S, Kim YI, Min HY, Kim DJ, Lee DS, Cho D, Hwang YI, Hwang DH, Park SH, Ahn HK, Chang KY, Kim YB, Lee WJ. CM1, a possible novel activation molecule on human lymphocytes. Immunol Lett. 2000. 74:95–102.

2. Kang JS, Kim D, Kim YI, Lee WJ, Chang KY. Development of tumor screening ELISA kit by using novel tumor antigen, CM1. Immune Netw. 2005. 5:124–128.

3. Lee YS, Kim YS, Kim D, Hur DY, Kang JS, Kim YI, Hahm ES, Cho DH, Hwang YI, Lee WJ. CM1 ligation induces apoptosis via Fas-FasL interaction in Ramos cells, but via down-regulation of Bcl-2 and subsequent decrease of mitochondrial membrane potential in Raji cells. Immune Netw. 2006. 6:59–66.

4. Kim D, Hur DY, Kim YS, Lee K, Lee Y, Cho D, Kang JS, Kim YI, Hahm E, Yang Y, Yoon S, Kim S, Lee WB, Park HY, Kim YB, Hwang YI, Chang KY, Lee WJ. CM1 ligation initiates apoptosis in a caspase 8-dependent manner in Ramos cells and in a mitochondria-controlled manner in Raji cells. Hum Immunol. 2002. 63:576–587.

5. Kim YS, Park GB, Choi YM, Kwon OS, Song HK, Kang JS, Kim YI, Lee WJ, Hur DY. Ligation of centrocyte/centroblast marker 1 on Epstein-Barr virus-transformed B lymphocytes induces cell death in a reactive oxygen species-dependent manner. Hum Immunol. 2006. 67:795–807.

6. Giallongo A, Feo S, Showe LC, Croce CM. Isolation and partial characterization of a 48-kDa protein which is induced in normal lymphocytes upon mitogenic stimulation. Biochem Biophys Res Commun. 1986. 134:1238–1244.

7. Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells. identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991. 30:1682–1691.

8. Marín JM, Gracia-Lor E, Sancho JV, López FJ, Hernández F. Application of ultra-high-pressure liquid chromatography-tandem mass spectrometry to the determination of multi-class pesticides in environmental and wastewater samples. Study of matrix effects. J Chromatogr A. 2009. 1216:1410–1420.

9. Petrovic M, Gros M, Barcelo D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J Chromatogr A. 2006. 1124:68–81.

10. Pancholi V. Multifunctional alpha-enolase. its role in diseases. Cell Mol Life Sci. 2001. 58:902–920.
11. Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. J Rheumatol. 2000. 27:109–115.
12. Saulot V, Vittecoq O, Charlionet R, Fardellone P, Lange C, Marvin L, Machour N, Le Loët X, Gilbert D, Tron F. Presence of autoantibodies to the glycolyticenzymealphaenolase in sera from patients with earlyrheumatoid arthritis. Arthritis Rheum. 2002. 46:1196–1201.

13. Li J, Ny A, Leonardsson G, Nandakumar KS, Holmdahl R, Ny T. The plasminogen activator/plasmin system is essential for development of the joint inflammatory phase of collagen type II-induced arthritis. Am J Pathol. 2005. 166:783–792.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download