Abstract
Background
Nanoparticles (NPs) prepared from biodegradable polymers, such as poly (D,L-lactic acid-co-glycolic acid) (PLGA), have been studied as vehicles for the delivery of antigens to phagocytes. This paper describes the preparation of antigen-loaded PLGA-NPs for efficient cross-priming.
Methods
NPs containing a similar amount of ovalbumin (OVA) but different sizes were produced using a micromixer-based W/O/W solvent evaporation procedure, and the efficiency of the NPs to induce the cross-presentation of OVA peptides were examined in dendritic cells (DCs). Cellular uptake and biodistribution studies were performed using fluorescein isothiocyanate (FITC)-loaded NPs in mice.
Results
The NPs in the range of 1.1~1.4µm in size were the most and almost equally efficient in inducing the cross-presentation of OVA peptides via H-2Kb molecules. Cellular uptake and biodistribution studies showed that opsonization of the NPs with mouse IgG greatly increased the percentage of FITC-positive cells in the spleen and lymph nodes. The major cell type of FITC-positive cells in the spleen was macrophages, whereas that of lymph nodes was DCs.
Nano- and microparticles prepared from biodegradable polymers, such as PLGA and poly(L-lactic acid) (PLG) have been studied extensively for the sustained delivery of therapeutic agents including DNA, proteins and low molecular weight pharmacological agents (1,2). Nano- and microparticles prepared from PLGA and PLG have also been studied as a vehicle to deliver encapsulated antigens to phagocytes (3-5). PLGA-microspheres have been shown to deliver antigens to APCs efficiently and generate Th1-type immune responses even against poor immunogens (6,7). Antigens encapsulated with PLGA were at least 100 times more effectively presented by MHC-I molecules on DCs compared to the soluble form of the antigens (8). One of the major advantages of encapsulation would also be the protection of the antigen or the drug from premature release and degradation (1).
Recent studies have shown that PLGA particle-mediated antigen delivery enhances and prolongs the MHC class I-restricted presentation of the exogenous antigens (cross-presentation) in DCs (5,9). The uptake of PLGA-particles by phagocytes is influenced by a range of factors, such as the size, surface properties and concentration in the medium (10-12). Particles between 3~10µm in size were preferentially internalized by APCs (13,14). Although many studies have reported on the uptake of PLGA particles by phagocytes, relatively little is known about the relationship between the particle size and cross-presentation-inducing ability of the particles. The in vivo specific targeting of PLGA-encapsulated antigens to professional APCs is essential for the efficient induction of antigen-specific CD8 T cell responses. Although neutrophils are the most aggressive phagocytic cells in the blood, they do not serve as professional APCs. Because professional APCs express receptors for the Fc region of IgG, opsonization of PLGA particles with IgG might be a way of preferentially targeting particles to professional APCs.
In the present study, OVA-containing PLGA particles with different sizes but containing similar amounts of OVA were generated and the cross-presentation-inducing activities in DCs were compared. In addition, the effect of opsonizing the PLGA particles with IgG on the cellular uptake and biodistribution in mice was examined.
A T cell hybridoma, B3Z86/90.14 (B3Z), was kindly provided by Dr. Nilabh Shastri (University of California, Berkeley, CA) and T cell hybridomas, CD8 OVA1.3, were kindly provided by Dr. Clifford V. Harding (Case Western Reserve University, Cleveland, OH) (15,16). Bone marrow (BM)-derived DCs were generated from mouse bone marrow cells, as described previously (8). Briefly, BM cells obtained from the femurs of BALB/c mouse were cultured in a 6-well plate (5×106/well) in a culture medium supplemented with 200 U/ml rmGM-CSF. At days 3 and 4 from the initiation of culture, the non-adherent cells were discarded by replacing the culture medium with fresh medium containing the cytokines after gentle shaking. The DCs were harvested by gentle pipetting at day 6.
Particles containing OVA were prepared, as described previously with minor modifications (12). Briefly, 400µl of OVA (100 mg/ml) was added to 2 ml of ethyl acetate containing 200 mg of poly(lactic-co-glycolic acid) (PLGA, Sigma-Aldrich). The mixture was then stirred at 20,000 rpm (Homogeniser, IKA, Japan) in the presence or absence of simultaneous sonication (Ultrasonic bath: 500 W, 30 kHz, BRANSON). After 3 min of emulsification, 8 ml of an aqueous solution of polyvinyl alcohol (PVA, 5%) was added to the w/o emulsion to form a w/o/w double emulsion, which was stirred for 5 min. To solidify the nanoparticles, the organic solvent was evaporated by stirring the double emulsion with 200 ml of an aqueous solution of 0.1% PVA at 500 rpm for 2 h. For the complete removal of ethyl acetate, the dispersion of nanoparticles was concentrated to approximately half the volume using a rotary evaporator at 40℃. The resulting nanoparticles were centrifuged at 3,000 rpm for 20 min, and washed twice with phosphate-buffered saline (PBS). The mean size of the particles was measured using a particle size analyzer (ELS-Z, Otsuka, Japan). The OVA concentration was determined using a micro-bicinchoninic acid assay kit (Pierce, Rockford, IL) after lysing the nanoparticles in a lysis buffer containing 0.1% SDS and 0.1 N NaOH. Nanoparticles containing both OVA and fluorescein isothiocyanate (FITC) were prepared by adding FITC (final, 5 mg/ml) to ethyl acetate along with PLGA (final, 5%). The surface morphology of the formulated NPs was visualized by scanning electron microscopy (LEO-1530, Carl Zeiss, Germany).
OVA-specific mouse IgG (mIgG) was attached covalently to the nanoparticles using (1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide) (EDC, Pierce, Rockford, IL, USA) as previously described (12). Briefly, 4.5µg of EDC was added to a 360µl mixture of 400µg nanoparticles (as OVA concentration) and 400µg mIgG. The reaction mixture was incubated for 2 h by tapping at room temperature. An excess linking reagent and soluble byproducts were separated by centrifugation at 15,000 rpm for 10 min, and the nanoparticles were washed three times with 1 ml PBS, pH 7.4.
The DCs were added to a 96-well microtiter plate (1×105/well) and were then added with the nanoparticles. The plate was incubated for 2 or 18 h at 37℃,washed twice with 300 µl/well of pre-warmed PBS and fixed with 100µl/well of ice-cold 1.0% paraformaldehyde for 5 min at room temperature. The plate was then washed three times with 300 µl/well of PBS. Class I MHC-complexed OVA peptide quantities were assessed using LacZ T cell activation assays or by IL-2 secretion assays after culturing the paraformaldehyde-fixed DCs with B3Z cells (2×105/well) for 4 h or with CD8 OVA1.3 cells (2×105/well) for 18 h, as described previously (8,17).
Four milligrams of OVA-NPs were dispersed in 4 ml of PBS (pH 7.2) containing 0.02% sodium azide. The suspension was then aliquoted to a 500µl/microfuge tube, and the microfuge tubes were kept at 37℃ or 4℃. At the indicated days, the microfuge tubes were centrifuged at 15,000 rpm for 10 min, and the supernatants were transferred to new tubes. The supernatants were filtered through a membrane filter (pore size 0.22µm), and then the protein content was analyzed using a micro-bicinchoninic acid assay kit (Pierce).
C57BL/6 mice were injected i.p. with 100µg of NPs containing both OVA and FITC. The mice were sacrificed 2 h later, and mononuclear cell suspensions were prepared from the spleens and lymph nodes (popliteal, inguinal, mesenteric, and axillary). The single cell suspensions were fixed with paraformaldehyde and analyzed for FITC-positive cells by flow cytometry, or stained with phycoerythrin-labeled anti-mouse CD11b, CD11c, or Gr-1 monoclonal antibodies (BD pharmingen, San Diego, CA), after blocking the FcR-binding anti-CD16/CD32 monoclonal antibody (clone 2.4G2), as described previously (18). Flow cytometry was performed on a FACS Cantor (Becton-Dickinson).
Table I lists the different formulation conditions used to prepare nano- and microparticles along with their mean particle size, polydispersity index, zeta potential and protein loading efficiency. As expected, increasing the homogenization speed resulted in smaller sized NPs. The application of sonication energy (500 W, 30 kHz) during homogenizing of the two phases, the ethyl acetate phase containing PLGA and water phase containing OVA, further decreased the size of the resulting NPs. The mean OVA content was determined using a micro-bicinchoninic acid assay kit after lysing the NPs in a lysis buffer, and was 22.0~24.8%. The surface morphology of the NPs was visualized by scanning electron microscopy, and Fig. 1A shows a representative image of the NPs with an average size of 1.1µm. The stability of the NPs was examined by assessing the amount of soluble OVA released from the NP. Fig. 1B gives a representative experimental result, which was performed using the NPs with a mean size of 1.1µm. The OVA entrapped within the NPs was not released from the NPs for at least one week from the NPs if the NPs were stored at 4℃.
To examine the relationship between the NP size and cross-presentation-inducing activity, the NPs were incubated with BM-DCs for 2 h and the DCs were fixed with paraformaldehyde. The amounts of H-2Kb-OVA peptides complexes on DCs were assessed using OVA-specific CD8 T cell hybridomas, B3Z cells, which express β-galactosidase in response to T cell receptor (TCR) stimulation. As shown in Fig. 2, DCs phagocytosed NPs in the range of 1.11~1.44µm in size were almost equally efficient in cross-presenting OVA peptides via H-2Kb molecules. The cross-priming-inducing activity of NPs decreased dramatically when the mean size of the NPs was reduced to 0.56µm. Therefore, NPs with the average size of 1.11µm were chosen for the experiments described hereafter.
The NPs (average size, 1.11µm) were opsonized with mouse IgG, and the amounts and time kinetics of OVA peptide presentation were examined in BM-DCs. In this experiment, the amounts of H-2Kb-OVA peptide complexes on DCs were assessed using OVA-specific CD8 T cell hybridomas, CD8 OVA1.3 cells, which express IL-2 in response to H-2Kb-OVA peptide (SIINFEKL) complexes. As shown in Fig. 3, DCs incubated with the NPs containing OVA (NP[OVA]) were much more efficient in cross-presenting OVA peptides than those incubated with soluble OVA. In particular, the DCs incubated with mouse IgG-opsonized NPs (mIgG-NP[OVA]) were much more potent in cross-presenting OVA peptides than those incubated with (NP[OVA]). Moreover, the duration of cross-presenting OVA peptides was also extended markedly in DCs phagocytosed mIgG-NP[OVA]. The DCs phagocytosed mIgG-NP[OVA] could present H-2Kb-OVA peptide (SIINFEKL) complexes for 24 h, whereas the DCs incubated with saturation amounts of synthetic OVA peptide, SIINFEKL, could present H-2Kb-SIINFEKL complexes for only 6 h.
Two hours after an intraperitoneal injection of a single dose of 100µg of FITC-loaded NPs, biodistribution analysis was performed using mononuclear cells isolated from the spleens and lymph nodes. As shown in Fig. 4, the percentage of FITC-positive cells in the spleen and lymph node were increased dramatically when the mice were injected with mouse IgG-opsonized FITC-loaded NPs, compared to the mice injected with unopsonized FITC-loaded NPs. Most of the FITC-positive cells were in the spleen (approximately 20% in the spleen vs. approximately 0.8% in the lymph nodes). To examine the cell type that is positive for FITC, the mononuclear cells isolated from the spleens and lymph nodes were stained with PE-conjugated anti-mouse CD11b, CD11c, or Gr-1 monoclonal antibodies. As shown in Fig. 5, macrophages were the major cell type of FITC-positive cells in the spleen, whereas DCs were the major type in the lymph nodes.
One of the main issues addressed in the present study is the elucidation of the optimal size of PLGA particles for the induction of the most potent cross-priming for the antigens entrapped inside the particles. Therefore, the present study developed methods to produce PLGA-NPs with various mean sizes. This method is based on the micromixer-based W/O/W solvent evaporation procedure but with a slight modification, such as adding sonication energy during the homogenization procedure. PLGA-NPs containing almost the same amount of ovalbumin (OVA) were generated but with different mean sizes. The efficiency of the NPs to induce cross-presentation of the OVA peptides were examined in DCs. The NPs in the range of 1.1~1.4µm in size were the most potent and almost equally efficient in inducing the cross-presentation of OVA peptides via H-2Kb molecules in DCs. The size of the PLGA-NPs optimal for cross-priming is markedly smaller than the size of the PLGA-NPs that were reported by other researchers to be optimal for phagocytosis by APCs. It PLGA-NPs between 3~10µm in size were optimal for uptake by APCs (13,14).
One of the unique features of PLGA-NPs is the sustained release of the entrapped antigens. In addition, encapsulation of the antigen with PLGA would be the protection of the antigen from rapid degradation in the body (1). Therefore, particles prepared from PLGA have been studied extensively for the sustained delivery of therapeutic agents including DNA, proteins and low molecular weight pharmacological agents used as vehicles to facilitate antigen delivery to phagocytes (1,2). Moreover, PLGA-NPs are easily conjugated with IgG. Coating the PLGA-NPs with mouse IgG is expected to decrease the uptake of NPs by neutrophils while increasing the uptake of NPs by Fcγ receptor (FcγR)-positive cells, such as DCs and macrophages. Therefore, IgG-opsonized OVA-containing PLGA NPs with a average size of 1.1µm would be the best vehicle for delivering protein antigens to professional APCs in vivo. This hypothesis was confirmed by the experiments described in this manuscript. Throughout this study, IgG-opsonized OVA-containing PLGA-NPs were found to be the superior means for inducing the cross-presentation of OVA peptides in DCs.
The reason this study focused on the cross-presentation-inducing activities of the PLGA-NPs in the present study is that the importance of enhancing cross-presentation, which was first demonstrated in the generation of CTL responses to minor histocompatibility antigens (19), has now been demonstrated to be an essential mechanism for the generation of CTL responses against tumor cells, transplanted cells, bacteria and even viruses (20-23). The present study also showed that DCs phagocytosed IgG-opsonized OVA-containing PLGA-NPs can present OVA peptides for the most extended time periods, compared to any other means used in this study. In addition, IgG-opsonized OVA-containing PLGA-NPs were captured efficiently by professional APCs in the spleen and lymph nodes when injected intraperitoneally.
Overall, these results show that IgG-opsonized PLGA-NPs with a mean size of 1.1µm would be the choice of biodegradable carriers for the targeted-delivery of protein antigens for cross-priming in vivo.
Figures and Tables
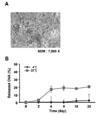 | Figure 1Release profile of OVA from OVA-loaded NPs. (A) Scanning electron microscopy showing the surface morphology of NPs with a mean size of 1.1µm. (B) Four milligrams of the OVA-NPs were dispersed in 4 ml of PBS containing 0.02% sodium azide, and then were kept at 37℃, or 4℃. At the indicated days, the supernatants were collected after centrifugation, and then the protein contents were analyzed using a micro-bicinchoninic acid assay. |
 | Figure 2Effects of the size of NPs on the cross-presentation. DCs were incubated with NPs with different mean sizes for 2 h, washed with PBS, fixed with paraformaldehyde, and the amounts of OVA peptides presented on MHC class I molecules were assessed by a LacZ T cell activation assay using OVA specific CD8 T cell hybridomas, B3Z cells. |
 | Figure 3Time-kinetics for the cross-presentation of OVA peptides. DCs were incubated with soluble OVA (50µg/ml), OVA-loaded NPs (50µg/ml), IgG-opsonized OVA-loaded NPs (50µg/ml), or 1 nM synthetic OVA peptide (SIINFEKL) for 2 h. The DCs were then washed with PBS, and were cultured in a medium containing 10% fetal bovine serum. At the indicated time points, the DCs were fixed with paraformaldehyde, and the amounts of OVA peptides presented on MHC class I molecules were assessed by a LacZ T cell activation assay using OVA specific CD8 T cell hybridomas, B3Z cells. |
 | Figure 4The uptake and biodistribution of NPs injected into the mouse peritoneum. C57BL/6 mice were injected i.p. with 100µg of NPs containing both OVA and FITC. Mice were sacrificed 2 h later, and mononuclear cell suspensions were prepared from the spleens (A) and lymph nodes (popliteal, inguinal, mesenteric, and axillary) (B). The single cell suspensions were fixed with paraformaldehyde and analyzed for FITC-positive cells by flow cytometry. |
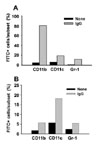 | Figure 5Cell type analysis for the FITC-positive cells in the spleen and lymph nodes. C57BL/6 mice were injected i.p. with 100µg of NPs containing both OVA and FITC. The mice were sacrificed 2 h later, and mononuclear cell suspensions were prepared from the spleens (A) and lymph nodes (B), stained with phycoerythrin-labeled anti-mouse CD11b, CD11c, or Gr-1 monoclonal antibodies, and analyzed for FITC-positive cells by flow cytometry. |
ACKNOWLEDGEMENTS
This study was supported by the research grant of the Chungbuk National University in 2009.
References
1. Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976. 143:1283–1288.

2. Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008. 25:1815–1821.

3. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004. 22:2406–2412.

4. Gerelchuluun T, Lee YH, Lee YR, Im SA, Song S, Park JS, Han K, Kim K, Lee CK. Dendritic cells process antigens encapsulated in a biodegradable polymer, poly(D,L-lactide-co-glycolide), via an alternate class I MHC processing pathway. Arch Pharm Res. 2007. 30:1440–1446.

5. Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994. 153:4925–4933.
6. Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001. 19:47–64.

7. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.

8. Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005. 57:391–410.

9. Johansen P, Men Y, Merkle HP, Gander B. Revisiting PLA/PLGA microspheres: an analysis of their potential in parenteral vaccination. Eur J Pharm Biopharm. 2000. 50:129–146.

10. Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992. 89:6020–6024.

11. Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release. 2007. 120:18–26.

12. Lee JK, Lee MK, Yun YP, Kim Y, Kim JS, Kim YS, Kim K, Han SS, Lee CK. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001. 1:1275–1284.

13. Lee YH, Lee YR, Kim KH, Im SA, Song S, Lee MK, Kim Y, Hong JT, Kim K, Lee CK. Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells. Int Immunopharmacol. 2011. [Epub ahead of print].

14. Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005. 105:3951–3955.

15. Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008. 125:193–209.

16. Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by Poly(d,l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci. 1998. 87:1421–1427.

17. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–347.

18. Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002. 82:105–114.

19. Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006. 117:78–88.

20. Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999. 398:77–80.

21. Venkataprasad N, Coombes AG, Singh M, Rohde M, Wilkinson K, Hudecz F, Davis SS, Vordermeier HM. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine. 1999. 17:1814–1819.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download