Abstract
Background
Mycobacterium tuberculosis (Mtb) heparin binding hemagglutinin (HBHA) is an Ag known to evoke effective host immune responses during tuberculosis infection. However, the molecular basis of the host immune response to HBHA has not been fully characterized. In this study, we examined the molecular mechanisms by which HBHA can induce the expression of proinflammatory cytokines in macrophages.
Methods
HBHA-induced mRNA and protein levels of proinflammatory cytokines were determined in bone marrow-derived macrophages (BMDMs) using RT-PCR and ELISA analysis. The roles of intracellular signaling pathways for NF-κB, PI3-K/Akt, and MAPKs were investigated in macrophage proinflammatory responses after stimulation with HBHA.
Results
HBHA robustly activated the expression of mRNA and protein of both TNF-α and IL-6, and induced phosphorylation of NF-κB, Akt, and MAPKs in BMDMs. Both TNF-α and IL-6 production by HBHA was regulated by the NF-κB, PI3-K, and MAPK pathways. Furthermore, PI3-K activity was required for the HBHA-induced activation of ERK1/2 and p38 MAPK, but not JNK, pathways.
Mycobacterium tuberculosis (Mtb) or mycobacterial Ags initiate proinflammatory responses in mononuclear phagocytes by interaction with a variety of signaling molecules/machineries. The relationships between specific mycobacterial components and signaling pathways are crucial for the induction of appropriate host responses, such as mounting a protective immune response, during mycobacterial infection (1). One of the Mtb-Ags, heparin-binding hemagglutinin (HBHA), is important for adherence to epithelial cells (2). This protein can induce mycobacterial aggregation, hemagglutination, and binding to heparin sulfate proteoglycans through post-translational modifications in the C-terminal lysine-rich domain of the protein (3,4). In addition, HBHA protein reacts with sera from tuberculosis (TB) patients, but not from healthy individuals, suggesting potent immunogenicity during mycobacterial infections (4). Moreover, HBHA has been widely studied for its potential to elicit effective host immune responses against TB (5). However, little is known regarding the molecular mechanisms by which HBHA modulates host innate immune responses.
Upon mycobacterial infection, the engagement of mycobacteria with innate receptors in host cells leads to the activation of signaling pathways that then results in the induction of inflammatory cytokines and antimicrobial effectors (6). Importantly, the mycobacterial cell wall contains a number of proinflammatory TLR2 ligands, including lipoproteins and lipoarabinomannan, and induces activation of the MAPKs and NF-κB pathways (1,7). Subsequent innate responses likely serve to decrease the inoculum size during the initial infection and drive an adaptive immune response (6). The MAPK pathways play an important role in enhancing antimycobacterial activity and production of immune effector molecules, including TNF-α (1). The proinflammatory cytokine TNF-α plays a crucial role in defense against mycobacterial infection including phagocytosis, intracellular killing, stimulating T cell activation, and granuloma formation (8,9). Our previous studies have reported that the proinflammatory cytokines TNF-α, IL-1β, and IL-6 are up-regulated in monocytes and macrophages isolated from the early stages of active pulmonary TB patients (10,11). In addition, it was shown that activation of ERK1/2 and p38 MAPK, proinflammatory cytokine secretion, and apoptotic activities were greater in monocytes or neutrophils from TB patients compared with healthy control subjects (11-13).
Although previous studies of TB and mycobacterial Ags have contributed to marked advances in our knowledge of the host protective immune responses, a number of critical questions are not well understood. Here, we present the intracellular signaling pathways activated by HBHA stimulation in murine bone marrow-derived macrophages (BMDMs). First, we determined whether HBHA Ag induced TNF-α and IL-6 production in BMDMs. We then examined the roles of PI3-K and MAPKs:p38, JNK, and ERK1/2 in BMDMs. We also found that the PI3-K and MAPK pathways contribute to an induction of HBHA-induced TNF-α and IL-6 production in BMDMs. To the best of our knowledge, this is first study to investigate the molecular mechanisms underlying the regulation of innate immune responses induced by HBHA.
Wild-type C57BL/6 mice were purchased from KOATECH (Pyungtek, Korea). All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee, Chungnam National University. BMDMs were differentiated for 5~7 d in media containing M-CSF (R&D systems, Minneapolis, MN, USA), as described previously (1). The culture media contain DMEM (Gibco-BRL, Gaithersburg, NY, USA) with 10% heat-inactivated FBS (Gibco-BRL), 1 mM sodium pyruvate, 50 U/ml penicillin, 50µg/ml streptomycin and 5×105 M β-mercaptoethanol, sodium pyruvate, non-essential amino acids, penicillin G (100 IU/ml), and streptomycin (100µg/ml). The mouse macrophage cell line RAW264.7 (American Type Culture Collection; ATCC TIB-71) was maintained in complete medium (DMEM with 10% FBS) with sodium pyruvate, non-essential amino acids, penicillin G (100 IU/ml), and streptomycin (100µg/ml).
All reagents and chemicals were purchased from Sigma-Aldrich except those noted below, which were obtained from the indicated suppliers:BAY 11-7082, LY294002, Wortmannin, U0126, SB203580, and SP600125 were purchased from Calbiochem (San Diego, CA, USA). Specific Abs against total Akt, phospho-Akt (Ser473), phospho-IκB-α (Ser32/36), phospho-ERK1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), and phospho-JNK (Thr183/Tyr185) were purchased from Cell signaling Technology (Cell signaling, Beverly, MA, USA). Anti-β-actin (I-19), anti-IκB-α (C-21), and anti-phospho-NF-κB p65 (C-20) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). DMSO (Sigma, St. Louis, MO, USA) was added to cultures at 0.1% (v/v) as a solvent control. The dominant negative (DN)-p38, DN-MEK1 and DN PI3-K p110α constructs were a kind gift from Dr. Eui-Ju Choi (Korea University, Seoul, Korea). Cells were transfected using LipofectAMINE as indicated by the manufacturer (Invitrogen, Carlsbad, CA, USA).
Methylated, recombinant HBHA was expressed in M. smegmatis (rMS-HBHA), as previously described with slight modifications (14,15). Purified recombinant HBHA was dialyzed against PBS (pH 7.2), applied to a column with immobilized polymyxin B (Detoxi-Gel Endotoxin Removing Gel; Pierce, Rockford, IL, USA) to reduce the level of endotoxin, filter-sterilized, and then frozen at -20℃. Protein concentrations were estimated using the bicinchoninic acid protein assay kit (Pierce), with BSA as the standard. The endotoxin content in HBHA was 0.017 ng/µg protein, as determined by the limulus-amebocyte-lysate test (QCL1000; BioWhittaker, Walkersville, MD, USA).
BMDMs were treated for the times indicated and processed for analysis by a sandwich ELISA and Western blot, as previously described (1). For the sandwich ELISA, cytokine levels in cell culture supernatants were determined using Duoset Ab pairs (BD PharMingen, San Diego, CA, USA) for the detection of TNF-α and IL-6, as described (1). All assays were performed as recommended by the manufacturers. For Western blot analysis, primary Abs were used at a 1:1,000 dilution. The blots were then detected by the ECL detection system according to the recommended procedure (Amersham-Pharmacia, Freiburg, Germany).
For semi-quantitative RT-PCR analysis, total RNA was extracted from BMDMs (1×106) using TRIzol (Invitrogen), as previously described (11). For PCR amplifications of specific cDNAs derived from TNF-α, IL-6, and β-actin mRNAs, three sets of sense and antisense oligonucleotides were used. Primer sequences were as follows:mTNF-α (forward:5'-CGGACTCCGCAAAGTCTAAG-3', reverse:5'-ACGGCATGGATCTCAAAGAC-3'), mIL-6 (forward:5'-GGAAATTGGGGTAGGAAGGA-3', reverse:5'-CCGGAGAGGAGACTTCACAG-3'), and β-actin (forward:5'-TCATGAAGTGTGACGTTGACATCCGT-3', reverse:5'-CCTAGAAGCATTTGCGGTGCACGATG-3'). PCR amplification was performed with an initial denaturing step at 95℃ for 5 min, followed by 30 cycles of denaturation (95℃, 1 min), annealing (58℃, 1 min), and extension (72℃, 1 min), then a further extension at 72℃ for 10 min. The PCR products were electrophoresed in 1.5% agarose gels in the presence of ethidium bromide, visualized by ultraviolet fluorescence, and recorded by a digital camera connected to a computer (SYNGENE, Gene Genius Imaging System).
For NF-κB translocation analysis, BMDMs were treated with HBHA for 1 h and then fixed with 4% (w/v) paraformaldehyde in PBS for 10 min at room temperature (RT). Cells were then permeabilized with 0.25% (v/v) Triton X-100 in PBS for 15 min at RT and blocked for 1 h in PBS containing 3% BSA. The cells were immunostained with rabbit anti-mouse NF-κB p65 (1:200 dilution) for 2 h at RT. After washing with PBS, cells were incubated with 1:200 dilutions of anti-rabbit immunoglobulin-conjugated AlexaFluor 488 (Molecular Probes, Eugene, OR, USA) for 1 h at RT. Nucleus were visualized by incubation with DAPI (1µg/ml, Sigma). The nuclear translocation of NF-κB p65 was visualized using a Olympus BX51 fluorescence microscope (DP ver. 1.2.1.108, Olympus, Japan).
Several mycobacterial Ags, including methylated HBHA, play an essential role in the induction of immunogenicity during tuberculous pleurisy infection (10-12). We first examined whether purified mycobacterial HBHA induced the expression of TNF-α and IL-6 mRNA in BMDMs. Semiquantitative RT-PCR analysis showed that HBHA significantly induced the expression of TNF-α and IL-6 mRNA after 3 h of stimulation in BMDMs. Peak expression of TNF-α and IL-6 mRNA occurred 6 h after stimulation with HBHA (Fig. 1A). In addition, secretion of TNF-α and IL-6 protein was assayed in BMDMs after HBHA stimulation. Treatment of BMDMs with HBHA significantly induced production of TNF-α and IL-6 after 6 h with a peak at 18 h (Fig. 1B). These data demonstrate that HBHA strongly induces proinflammatory cytokine expression by BMDMs.
NF-κB is a central regulator of inflammatory responses, and activation of NF-κB is required for the transcriptional induction of many proinflammatory mediators involved in innate immunity, including cellular adhesion molecules, cytokines, and growth factors (16). After stimulation with HBHA for the times indicated, expression of IκB-α was dramatically attenuated after 15~30 min, whereas IKKα/β phosphorylation was strongly induced after 15~30 min (Figs. 2A and B). The MAPK pathways are crucial for macrophage signaling during mycobacterial infection (17,18). We next examined whether HBHA induced MAPK activation in BMDMs. HBHA stimulation induced MAPK (ERK 1/2, p38, and JNK) activation within 15 min of stimulation, with a peak at 15~30 min (Figs. 2A and C). These data indicate that HBHA robustly activates NF-κB and MAPK signaling pathways in macrophages.
We next investigated the role of NF-κB in HBHA-induced proinflammatory cytokine production by BMDMs. We first confirmed translocation of NF-κB p65 into the nucleus after stimulation with HBHA (Fig. 3A). Pretreatment of BMDMs with an NF-κB inhibitor (BAY 11-7082, an inhibitor of IκB phosphorylation) significantly attenuated production of TNF-α and IL-6 in a dose-dependent manner (Fig. 3B). Furthermore, pre-treatment of BMDMs with other NF-κB inhibitors (IKK-2 inhibitor and NF-κB activation inhibitor) significantly reduced TNF-α and IL-6 mRNA expression (data not shown). These results indicate a critical role for NF-κB in the modulation of HBHA-induced inflammatory responses.
In previous studies, we found a rapid phosphorylation of the PI3-K/Akt pathway in human monocytes after stimulation with the 30 kDa or purified protein derivative (PPD) of Mtb (19,20). However, the nature of PI3-K/Atk activation after HBHA stimulation remains unknown. We also investigated whether HBHA stimulation induces activation of the Akt pathway in BMDMs. As shown in Fig. 4A, HBHA induced phosphorylation of Akt within 5 min of stimulation and marked activation of Akt occurred within 2~8 h of HBHA stimulation.
To examine the role of the PI3-K/Akt pathway in HBHA-induced TNF-α and IL-6 production, BMDMs were pre-treated with LY294002 or Wortmannin, which are pharmacologic inhibitors of PI3-K, prior to HBHA stimulation. TNF-α and IL-6 mRNA and protein levels in the cells and supernatants, respectively, were then examined. As shown in Figs. 4B and C, pre-treatment with LY294002 or Wortmannin significantly inhibited TNF-α and IL-6 mRNA expression (Fig. 4B) and protein production (Fig. 4C) in HBHA-treated BMDMs. Furthermore, these data were confirmed using PI3-K p110α-DN. RAW264.7 cells were transfected with the PI3-K p110α-DN construct or mock control before stimulation by HBHA and then subjected to ELISA. A similar significant inhibition of TNF-α and IL-6 production was observed in RAW264.7 cells transfected with the PI3K p110α-DN mutant construct compared with the mock control vector (Fig. 4D). These data suggest that the PI3-K/Akt pathway is essential for regulation of HBHA-induced TNF-α and IL-6 production by macrophages.
We next investigated the possible involvement of the ERK 1/2, p38 MAPK, or JNK pathways in HBHA-induced proinflammatory responses. Cells were stimulated with HBHA and cultured for 6 or 18 h to assess mRNA expression and TNF-α and IL-6 production. As shown in Figs. 5A and B, HBHA-induced TNF-α and IL-6 expression and protein production were significantly inhibited in BMDMs pretreated with specific MAPK inhibitors [inhibitors of MEK (U0126, PD98059), p38 MAPK (SB203580), and JNK (SP600125)].
Additionally, RAW264.7 cells were transfected with p38 MAPK-DN and MEK1-DN mutant constructs or a mock control vector prior to stimulation by HBHA. We observed a significant inhibition of TNF-α and IL-6 production in RAW264.7 cells transfected with the p38 MAPK-DN or MEK1-DN mutant construct compared with the mock control vector (Fig. 5C). These results indicate that HBHA-induced TNF-α and IL-6 production is regulated by the MAPKs (ERK 1/2, p38 MAPK, and JNK) pathway in macrophages.
Mycobacteria and mycobacterial components are potent activators of the PI3-K/Akt and MAPK pathways in macrophages (17,18,21-23). Previously, it was shown that the PI3-K and Akt pathways modulate the activation of ERK1/2 in response to Mtb or mycobacterial Triton X-100-solubilized protein Ag (24). We further determined whether HBHA-dependent PI3-K activation regulates the downstream MAPK pathways. To investigate the role of PI3-K in HBHA-induced MAPK activation, BMDMs were pretreated with Wortmannin or LY294002, stimulated with HBHA, and then assessed by Western blot analysis for ERK1/2, p38 MAPK, and JNK phosphorylation. As shown in Fig. 6, inhibition of PI3-K significantly attenuated HBHA-induced phosphorylation of Akt, as well as that of ERK1/2 and p38, in BMDMs. However, the activation of JNK was not modulated by inhibition of the PI3-K pathway. These results show that PI3-K/Akt activity is required for the HBHA-induced activation of ERK 1/2 and p38 MAPK, but not JNK.
In the present study, we demonstrate the molecular mechanisms underlying the regulation of the proinflammatory cytokines TNF-α and IL-6 by the PI3-K/Akt, p38, and ERK 1/2 MAPK pathways in response to the mycobacterial HBHA in murine macrophages. Mycobacteria and their components are potent activators of macrophages, which are able to produce proinflammatory mediators that are essential for innate defense and induction of the acquired immune response against mycobacteria (17,21,25,26). The mycobacterial HBHA, especially the methylated form, is known to be essential for effective protective immunity, which is comparable to that induced by vaccination with Bacillus Calmette-Guérin (27). Furthermore, previous studies reported that HBHA is significantly more sensitive than early secreted antigenic target-6 and more specific than PPD for the detection of latent TB infection (28). These observations strongly suggest that mycobacterial HBHA is involved in the pathogenesis of TB and might be useful in diagnosis. However, the role of HBHA in the induction of innate and inflammatory responses during mycobacterial infection has not been characterized.
We found that levels of the proinflammatory cytokines TNF-α and IL-6 were increased in BMDMs stimulated with HBHA. Vaccination with HBHA induces IFN-γ production by both CD4+ and CD8+ T cells, and CD8+ cytotoxic T cell responses (29). To the best of our knowledge, this is the first report that HBHA actively induces proinflammatory immune responses in macrophages. TNF-α, a critical proinflammatory cytokine, plays an essential role in the host protective immune responses against mycobacterial infection (30). We previously reported that mycobacterial Ags up-regulated the production of TNF-α, IL-1, and IL-6 in monocytes/macrophages isolated from early active TB patients, when compared with those from healthy controls (10,11). In addition, activation of ERK1/2 and p38 MAPK, proinflammatory cytokine secretion, and apoptotic activities were greater in monocytes or neutrophils from TB patients, compared with healthy control subjects (11-13). Combined with our findings, these reports suggest that HBHA contributes to the immunopathology of TB through active induction of proinflammatory cytokine release.
NF-κB is a central mediator of the inducible transcription of various proinflammatory genes in innate immune responses (31). Previous studies have shown that mycobacterial infection, or mycobacterial products, act through TLRs to trigger the MAPK pathways, leading to activation of transcription factors, including NF-κB (17,24,32). We found HBHA-induced increases in IKKα/β phosphorylation, as well as translocation of p65 from the cytosol to the nucleus. Importantly, inhibition of NF-κB significantly reduced HBHA-induced proinflammatory cytokine secretion. These data are consistent with the observed strong induction of proinflammatory cytokine expression by heat shock protein (33) and a 19-kDa lipoprotein (34) from Mtb. Taken together, our data suggest that NF-κB activation plays an essential role in HBHA stimulation of proinflammatory cytokine secretion by BMDMs.
Mycobacteria induce the PI3-K (35,36) and MAPK intracellular signaling cascades (1,21). In mycobacterial infections, the PI3-K pathway plays a role in human monocyte antimycobacterial activity (11,37). The MAPK signaling pathways are activated upon mycobacterial infection, and have been implicated in pathogenesis (18). The mycobacterial Ags 38 kDa and MTB12 can induce activation of ERK1/2 and p38 MAPK, and subsequent cytokine secretion in monocytes from active pulmonary TB patients (11,38). Although HBHA is widely studied for its potential to trigger effective host immune responses against TB (5), little is known regarding the signaling mechanisms underlying proinflammatory cytokine secretion by macrophages. Here, we suggest that mycobacterial HBHA can induce intracellular signaling pathways, such as PI3-K and MAPK, that are required for production of TNF-α and IL-6 in BMDMs.
Previously, we reported that macrophage recognition of mycobacteria and their Ags via innate receptors resulted in the activation of the MAPK and PI3-K/Akt pathways in monocytes/macrophages (11,21,39). The PI3-K and MAPK pathways contribute to proinflammatory cytokine secretion in response to the PPD and 30 kDa Ag of Mtb in human monocytes (19,20). Additionally, the PI3-K/Akt and ERK1/2 pathways are important for TNF-α expression in human monocyte-derived macrophages after treatment with Mtb H37Rv and Triton X-100-solubilized protein purified from Mtb (24). Interestingly, HBHA elicited a biphasic response, with an early peak of phospho-Akt observed after 5 min of stimulation, followed by a drop at 30~60 min and then a steady increase up to 8 h. These data are partly consistent with previous studies in which PI3-K/Akt showed a similar biphasic activation, i.e., by Francisella infection in murine macrophages (40) and LPS or α-lipoic acid in human monocytic THP-1 cells (41,42). Currently, the mechanisms by which phosphorylation of Akt at Ser473 are modulated during the early and late phases after stimulation of HBHA are not known. Future studies will likely elucidate the molecular mechanisms by which Akt phosphorylation is modulated and the exact roles of Akt phosphorylation during the early and late phases after HBHA stimulation.
Our results also demonstrate that PI3-K is a necessary upstream activator of the p38 and ERK1/2 MAPK pathways in BMDMs after stimulation with HBHA. Crosstalk between PI3-K/Akt and MEK-ERK pathways in different cell types has been demonstrated (43). Previously, it was reported that the PI3-K pathway is an upstream signal for ERK1/2 MAPK activation (24). However, this study did not determine how important HBHA is in proinflammatory innate responses or in the pathogenesis of TB. However, our data suggest a potential role for HBHA in the proinflammatory response to mycobacterial infection, since HBHA is a strong stimulator of proinflammatory cytokine secretion and an NF-κB activator. Recently, Jung et al. demonstrated that HBHA up-regulates the proinflammatory cytokines IL-6, IL-12, IL-1β, TNF-α, and CCR7 in dendritic cells (44). In the same study, HBHA-treated dendritic cells activated naïve T cells and polarized them to secrete IFN-γ (44). Other studies have shown that HBHA-induced IFN-γ production in alveolar and pleural lymphocytes is higher in pulmonary or pleural TB patients than in non-TB controls (45). Moreover, HBHA is strongly recognized by sera from pulmonary TB patients when compared with healthy controls (46). Taken together, our data strongly suggest that HBHA plays a potentially pleiotropic function in protective immunity (through IFN-γ-dependent protective immunity) and inflammatory responses (through inflammatory cytokine production) in TB.
Collectively, the data presented in this study provide a novel insight into molecular signaling by HBHA through activation of the NF-κB and PI3-K/Akt-p38-ERK1/2 MAPK pathways, which are responsible for the induction of pro-inflammatory responses during TB infection. In addition, our data reveal the key immunological processes induced by important human pathogens, including mycobacteria, and this information may assist in the rational design of more effective vaccines and adjuvants.
Figures and Tables
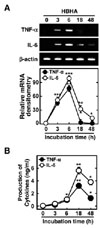 | Figure 1Kinetics of proinflammatory responses induced by HBHA in macrophages. BMDMs were stimulated with HBHA (1µg/ml) for the indicated periods of time. (A) Cells were harvested and semi-quantitative RT-PCR analysis of TNF-α and IL-6 mRNA level was performed. The relative densities of the expression levels were analyzed by densitometry. All densitometry values were normalized to that of β-actin mRNA. Top: representative gel images showing the products of RT-PCR analysis, Bottom: densitometric analysis. (B) The supernatants were harvested after the times indicated for assessment of TNF-α and IL-6 levels by ELISA. Mean results and densitometry values are depicted as mean±SD of three independent determinations. Significant differences (*p<0.05, **p<0.01, ***p<0.001) as compared with control cultures. |
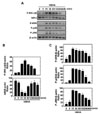 | Figure 2Kinetics of NF-κB and MAPK signaling pathway activation in macrophages after stimulation with HBHA. (A) BMDMs were stimulated with HBHA (1µg/ml) for the indicated periods of time. Cell lysates were harvested and subjected to Western blot analysis for phosphorylated IKK-α/β. The same blots were washed and blotted for IκB-α and phosphorylated MAPKs (p-ERK, p-p38, p-JNK). β-actin was probed as a loading control. Data are representative of three independent determinations with similar results. (B) Densitometric analysis of Western blot bands for phospho-IKK-α/β and IκB-α; both were normalized with β-actin. (C) Densitometric analysis of Western blot bands for phospho-ERK, p38, and JNK were normalized to β-actin. |
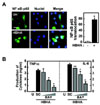 | Figure 3NF-κB activation is required for HBHA-induced TNF-α and IL-6 production by macrophages. (A) NF-κB translocation by HBHA. BMDMs were stimulated with HBHA (1µg/ml) for 1 h and immunostained with anti-NF-κB-p65 and anti-rabbit-Alexa Fluor 488. Left, representative images of three independent determinations with similar results. Scale bar=20µm. Right: quantification of data, NF-κ B-nuclear translocated cells were counted manually in DAPI-stained BMDMs. Data shown represent the means±SD of three independent samples, with each experiment including at least 200 cells scored in five random fields. (B) BMDMs were preincubated for 45 min with BAY 11-7082 (BAY; 0.3, 1, 3µM), prior to stimulation with HBHA (1µg/ml). Supernatants were harvested at 18 h and protein levels were determined by ELISA. Data represent the mean±SD of three independent determinations. Significant differences (*p<0.05, **p<0.01) as compared with HBHA-treated cells. U:unstimulated, SC:solvent control (0.1% DMSO). |
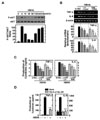 | Figure 4Effects of the PI3-K/Akt signaling pathways on HBHA-induced TNF-α and IL-6 expression by macrophages. (A) BMDMs were stimulated with HBHA (1µg/ml) for the times indicated. The cell lysates were then harvested and subjected to Western blot analysis for phosphorylated Akt. The same blots were washed and blotted for total Akt as the loading control. Top: representative gel image was shown, Bottom: densitometric analyses of Western blot bands for phospho-Akt was normalized to total Akt. Data are representative of three independent determinations with similar results. (B, C) Cells were pretreated with LY294002 (LY; 5, 10, 20µM) or Wortmannin (WM; 100, 200, 300 nM) for 45 min prior to stimulation with HBHA (1µg/ml) for 6 h (for B) or 18 h (for C). (B) Total RNA was purified and semi-quantitative RT-PCR was used to determine TNF-α and IL-6 expression in BMDMs. Top: representative gel image of three independent replicates was shown, Bottom: the relative densities of expression levels were analyzed by densitometry. All densitometry values were normalized to those of β-actin mRNA. (C) Supernatants were harvested at 18 h, and TNF-α and IL-6 levels were measured by ELISA. Data shown are the mean±SD of three determinations. (D) RAW264.7 cells were transfected with PI3-K p110α-DN or empty vector for 24 h prior to stimulation with HBHA (1µg/ml) for 18 h. The supernatants were then harvested and subjected to ELISA. Significant differences (*p<0.05, **p<0.01, ***p<0.001), compared with HBHA-treated cells or Mock control. U:unstimulated, SC:solvent control (0.1% DMSO). |
 | Figure 5MAPK signaling pathways are required for HBHA-induced TNF-α and IL-6 expression by macrophages. (A) BMDMs were pretreated with p38 inhibitor (SB203580; 1, 5, 10µM), MEK-1 inhibitor (U0126; 5, 10, 20µM), or JNK inhibitor (SP600125; 5, 20, 30µM) prior to stimulation with HBHA (1µg/ml). Cells were then harvested at 6 h, and total RNA purified and subjected to semi-quantitative RT-PCR analysis for assessment of TNF-α and IL-6 expression. Top: representative gel image of three independent replicates was shown, Bottom: the relative densities of expression levels were analyzed by densitometry. All densitometry values were normalized to those of β-actin mRNA. (B) BMDMs were pretreated with p38 inhibitor (SB203580, 10µM), MEK-1 inhibitor (PD98059 and U0126, 20µM), or JNK inhibitor (SP600125, 20µM) prior to stimulation with HBHA (1µg/ml). The supernatants from BMDMs were collected 18 h after stimulation of HBHA, and subjected to ELISA analysis for assessment of TNF-α and IL-6 production. Data shown are the mean±SD of three determinations. (C) RAW264.7 cells were transfected with p38 MAPK-DN, MEK-1-DN, or empty vector prior to stimulation with HBHA (1µg/ml) for 18 h. The supernatants from cells were harvested and subjected to ELISA for determination of TNF-α and IL-6 production. Significant differences (*p<0.05, **p<0.01, ***p<0.001) as compared with control cultures. U:unstimulated, SC:solvent control (0.1% DMSO), SB:SB203580, PD:PD98059, SP:SP600125. |
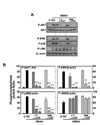 | Figure 6PI3-K/Akt pathway is the upstream signaling activator for p38 and ERK1/2 signaling pathways in macrophages after stimulation with HBHA. (A) BMDMs were incubated with LY294002 (LY; 5, 10, 20µM) or Wortmannin (WM; 100, 200, 300 nM) for 45 min prior to treatment with HBHA (1µg/ml). Cell lysates were then harvested (for p-Akt, at 5 min; for MAPKs, at 30 min), and subjected to Western blot analysis for p-Akt and MAPKs (p38, ERK, JNK). The same blots were stripped, washed, and re-probed with β-actin as a loading control. Data are representative of three independent determinations with similar results. (B) Densitometric analysis of the Western blot bands for p-Akt, p-ERK, p-p38, and p-JNK were normalized to total-Akt (for p-Akt) or β-actin (for MAPKs). Significant differences (*p<0.05, **p<0.01, ***p<0.001), compared with control cultures. U:unstimulated, SC:solvent control (0.1% DMSO). |
ACKNOWLEDGEMENTS
This study was financially supported by research fund of Chungnam National University in 2009. We thank Dr. Gang-Min Hur (Chungnam National University, Daejeon, Korea) and Eui-Ju Choi (Korea University, Seoul, Korea) for kind provision of constructs. The authors have no financial conflict of interest.
References
1. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria:branching out from Toll-like receptors. Cell Microbiol. 2007. 9:1087–1098.

2. Menozzi FD, Bischoff R, Fort E, Brennan MJ, Locht C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci U S A. 1998. 95:12625–12630.

3. Pethe K, Bifani P, Drobecq H, Sergheraert C, Debrie AS, Locht C, Menozzi FD. Mycobacterial heparin-binding hemagglutinin and laminin-binding protein share antigenic methyllysines that confer resistance to proteolysis. Proc Natl Acad Sci U S A. 2002. 99:10759–10764.

4. Menozzi FD, Rouse JH, Alavi M, Laude-Sharp M, Muller J, Bischoff R, Brennan MJ, Locht C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996. 184:993–1001.

5. Parra M, Pickett T, Delogu G, Dheenadhayalan V, Debrie AS, Locht C, Brennan MJ. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect Immun. 2004. 72:6799–6805.

6. Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis:myths and truth. Microbes Infect. 2008. 10:995–1004.
7. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999. 285:732–736.

8. Toossi Z. Cytokine circuits in tuberculosis. Infect Agents Dis. 1996. 5:98–107.
9. Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988. 140:3006–3013.
10. Lee JS, Song CH, Lim JH, Kim HJ, Park JK, Paik TH, Kim CH, Kong SJ, Shon MH, Jung SS, Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 2003. 132:443–449.

11. Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006. 74:2686–2696.

12. Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008. 10:741–754.

13. Alemán M, Schierloh P, de la Barrera SS, Musella RM, Saab MA, Baldini M, Abbate E, Sasiain MC. Mycobacterium tuberculosis triggers apoptosis in peripheral neutrophils involving toll-like receptor 2 and p38 mitogen protein kinase in tuberculosis patients. Infect Immun. 2004. 72:5150–5158.

14. Delogu G, Bua A, Pusceddu C, Parra M, Fadda G, Brennan MJ, Zanetti S. Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis. FEMS Microbiol Lett. 2004. 239:33–39.

15. Shin AR, Lee KS, Lee JS, Kim SY, Song CH, Jung SB, Yang CS, Jo EK, Park JK, Paik TH, Kim HJ. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin Vaccine Immunol. 2006. 13:869–875.

16. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994. 12:141–179.
17. Pathak SK, Bhattacharyya A, Pathak S, Basak C, Mandal D, Kundu M, Basu J. Toll-like receptor 2 and mitogen- and stress-activated kinase 1 are effectors of Mycobacterium avium-induced cyclooxygenase-2 expression in macrophages. J Biol Chem. 2004. 279:55127–55136.

18. Yadav M, Roach SK, Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 2004. 172:5588–5597.

19. Lee HM, Shin DM, Kim KK, Lee JS, Paik TH, Jo EK. Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis. J Clin Immunol. 2009. 29:46–56.

20. Jung SB, Song CH, Yang CS, Kim SY, Lee KS, Shin AR, Lee JS, Nam HS, Kim HJ, Park JK, Paik TH, Jo EK. Role of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in the secretion of tumor necrosis factor-alpha and interleukin-10 by the PPD antigen of Mycobacterium tuberculosis. J Clin Immunol. 2005. 25:482–490.

21. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection:the MAP kinases lead the way. Cell Microbiol. 2003. 5:133–142.

22. Darieva Z, Lasunskaia EB, Campos MN, Kipnis TL, Da Silva WD. Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J Leukoc Biol. 2004. 75:689–697.

23. Sendide K, Reiner NE, Lee JS, Bourgoin S, Talal A, Hmama Z. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria:regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005. 174:4210–4219.

24. Yang CS, Lee JS, Jung SB, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Differential regulation of interleukin-12 and tumour necrosis factor-alpha by phosphatidylinositol 3-kinase and ERK 1/2 pathways during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2006. 143:150–160.

25. Weir RE, Black GF, Dockrell HM, Floyd S, Fine PE, Chaguluka SD, Stenson S, King E, Nazareth B, Warndorff DK, Ngwira B, Crampin AC, Mwaungulu L, Sichali L, Jarman E, Donovan L, Blackwell JM. Mycobacterial purified protein derivatives stimulate innate immunity:Malawians show enhanced tumor necrosis factor alpha, interleukin-1beta (IL-1beta), and IL-10 responses compared to those of adolescents in the United Kingdom. Infect Immun. 2004. 72:1807–1811.

26. Wallis RS, Amir-Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins:the monocyte western blot. Proc Natl Acad Sci U S A. 1990. 87:3348–3352.

27. Temmerman S, Pethe K, Parra M, Alonso S, Rouanet C, Pickett T, Drowart A, Debrie AS, Delogu G, Menozzi FD, Sergheraert C, Brennan MJ, Mascart F, Locht C. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat Med. 2004. 10:935–941.

28. Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, Debrie AS, Doherty TM, Van Vooren JP, Locht C, Mascart F. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One. 2007. 2:e926.
29. Locht C, Hougardy JM, Rouanet C, Place S, Mascart F. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis (Edinb). 2006. 86:303–309.

30. Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001. 193:239–246.

31. Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008. 8:837–848.
32. Wang T, Lafuse WP, Zwilling BS. NFkappaB and Sp1 elements are necessary for maximal transcription of toll-like receptor 2 induced by Mycobacterium avium. J Immunol. 2001. 167:6924–6932.

33. Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005. 280:20961–20967.

34. Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001. 291:1544–1547.

35. Maiti D, Bhattacharyya A, Basu J. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001. 276:329–333.

36. Vanhaesebroeck B, Jones GE, Allen WE, Zicha D, Hooshmand-Rad R, Sawyer C, Wells C, Waterfield MD, Ridley AJ. Distinct PI(3)Ks mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999. 1:69–71.

37. Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001. 276:35482–35493.

38. Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 2006. 64:145–154.

39. Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003. 23:194–201.
40. Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006. 177:6317–6324.

41. Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002. 277:32124–32132.

42. Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007. 104:4077–4082.

43. Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996. 271:12133–12136.

44. Jung ID, Jeong SK, Lee CM, Noh KT, Heo DR, Shin YK, Yun CH, Koh WJ, Akira S, Whang J, Kim HJ, Park WS, Shin SJ, Park YM. Enhanced Efficacy of Therapeutic Cancer Vaccines Produced by Co-Treatment with Mycobacterium tuberculosis Heparin-Binding Hemagglutinin, a Novel TLR4 Agonist. Cancer Res. 2011. 71:2858–2870.

45. Place S, Verscheure V, de San N, Hougardy JM, Schepers K, Dirix V, Dediste A, Michel O, Drowart A, Allard SD, Doherty TM, Lecher S, Locht C, Mascart F. Heparin-binding, hemagglutinin-specific IFN-gamma synthesis at the site of infection during active tuberculosis in humans. Am J Respir Crit Care Med. 2010. 182:848–854.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download